Introduction
Fullerene (C60) is the shape of a soccer ball with pentagonal and hexagonal structure of carbon atoms, and the fullerene molecule with sixty carbon atoms was first made by Kroto and Smally.1 Fullerene (C60) is a carbon allotrope such as diamond and graphite, which has a stable structure that can withstand high heat and pressure, as results, has less reactivity and exhibits unique properties that absorb light and electricity well.2 Fullerenes are used in various fields3 and exhibit sensitive reactions owing to their fine structures. Therefore, they can be used as new fibers, catalysts, and sensors.4 Fullerene derivatives have received considerable attention, and various novel fullerene-based molecules have been reported.2 One of the simplest among them is the fullerene oxide [C60(O)n, (n≥1)]. In this molecule, the attached oxygen atom breaks the icosahedral symmetry of the C60 molecule and hence it affects to the properties and both the intra- and inter-molecular dynamics.5 The orientation and motion of the oxygen atoms in this molecule are of particular interest.6,7 Metal nanoparticles (NPs) act as excellent catalysts for reduction in the presence of NaBH4.8 The catalytic activity of silver metal particles depends significantly on their particle size. When particle size become smaller, not only their surface area of catalyst but also catalytic activity are increased.9 Over the past few years, silver nanoparticles as catalyst have gained immense attention since the activity of metal nanoparticles is extremely effective as heterogeneous catalysts.10,11 Ag NPs are less expensive than other reported metal nanoparticles and have similar catalyst efficiency.12 Generally, p-nitroaniline (NA) is primarily chosen industrially as a precursor to p-phenylenediamine, an important dye component. Nitroanilines (NAs) can cause long-term damage to the environment, especially if discharged into waterbod-ies.13 NAs are harmful for human health and the environment. Therefore, it is imperative to transform them into less toxic materials before discharging them into the environment.14 Reduction of nitroaromatic compounds using noble metal nanoparticle is an important method for conversion of toxic compound into environmental friendly meterials.15 In this research, ultrasonic irradiation was used for anchoring of Ag nanoparticles on fullerene oxide. Ultrasonic irradiation is an efficient method to obtain catalyst that holds uniform loading of silver nanoparticles. The effects of ultrasound irradiation is due to the acoustic cavitation phenomenon. When a liquid is irradiated with ultrasound, bubbles are created, these bubbles then grow and subsequent collapse, thus releasing the accumulated ultrasonic energy within a very short time.16-18
Experimental
Fullerene[C60] (TCI), m-chloroperoxybenzoic acid (Sigma-Aldrich), toluene (Samchun), silver nitrate[AgNO3] (Duksan), 2,3,4-nitroaniline (Sigma-Aldrich), sodium borohydride[NaBH4] (Sigma-Aldrich), and diethylene glycol[DEG] (TCI) were used in this experiment.
Initially, 40 mg of fullerene[C60] was dissolved in 100 ml of toluene, 196 mg of m-chloroperoxybenzoic acid was added to former solution. The mixture so obtained was refluxed for 5 h and then, toluene in the resulting solution was evaporated. The remaining solid material was washed with methanol to remove excess 3-chloroperoxybenzoic acid and dried in a vacuum oven for overnight.
15 mg of fullerene oxide[C60(O)n, (n≥1)] was dissolved into 22.5 ml of DEG, added 0.075 g of AgNO3 and 2.5 ml of distilled water. The resulting mixture solution was stirred for 30 min to ensure absorption of Ag nanoparticles on the surface of fullerene oxide[C60(O)n, (n≥1)].17 Ultrasonic irradiation was performed to mixed solution for 3 h (amplitude 70%) and was washed five times with distilled water by centrifugation. Then, the product was dried at 100°C for overnight.
Nitroaniline was reduced by the Ag nanoparticles which were anchored on the fullerene oxide[C60(O)n, (n≥1)]. 10 mg of NaBH4 was added to 50 mL of 0.25 mM 2-,3-,4-NA aqueous solutions with continuous stirring. And then, 3 mg of hybrid fullerene oxide[C60(O)n, (n≥1)] – silver nanoparticle composites as catalyst were added to various nitroanilines solution. The reduction of 2-,3-,4-nitroaniline was examined by obtaining the absorbance spectra of the solutions after every 5 min by UV-vis spectrophotometer, respectively.
The crystalline structure of hybrid fullerene oxide [C60(O)n, n≥1] – silver nanoparticle composites were examined by X-ray diffraction (XRD, D8-Advance, Bruker) equipped with Cu Ka radiation over 2θ range of −10° to 60°. Scanning electron microscopy (SEM, JEOL, JSM-7600F, Japan) was used to identify the surface morphology of the synthesized composite. The optical absorption spectra of samples were recorded using an UV-vis spectrophotometer (1601 PC, Shimadzu, Japan).
Results and Discussion
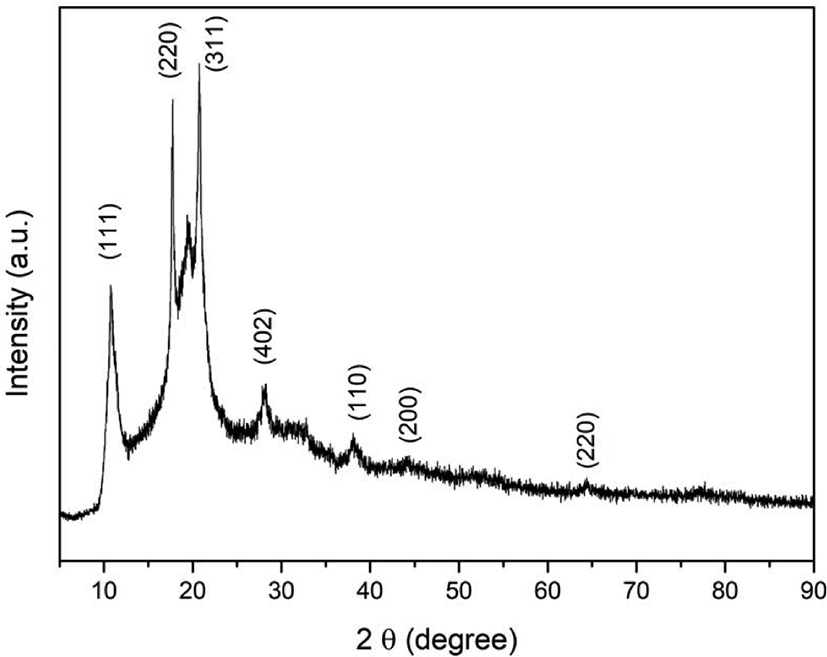
2 mg of fullerene oxide[C60(O)n, (n≥1)] – silver nanoparticle composites were examined for the XRD analysis and XRD patterns were shown in Figure 1. From the Figure 1, the diffraction peaks of fullerene oxide[C60(O)n, (n≥1)] – silver nanoparticle composites were observed at 2θ = 38.11°, 44.30°, and 64.44° as 2θ due to silver nanoparticles. This confirms the anchoring of Ag nanoparticles on the surface of the fullerene oxide[C60(O)n, (n≥1)] during the synthesis of the hybrid nanoparticle composites. The peak of 10.67°, 17.57°, and 20.64° as 2θ was corresponded to the (1 1 1) (2 2 0) and (3 1 1) plans of the fullerene oxide[C60(O)n, (n≥1)].
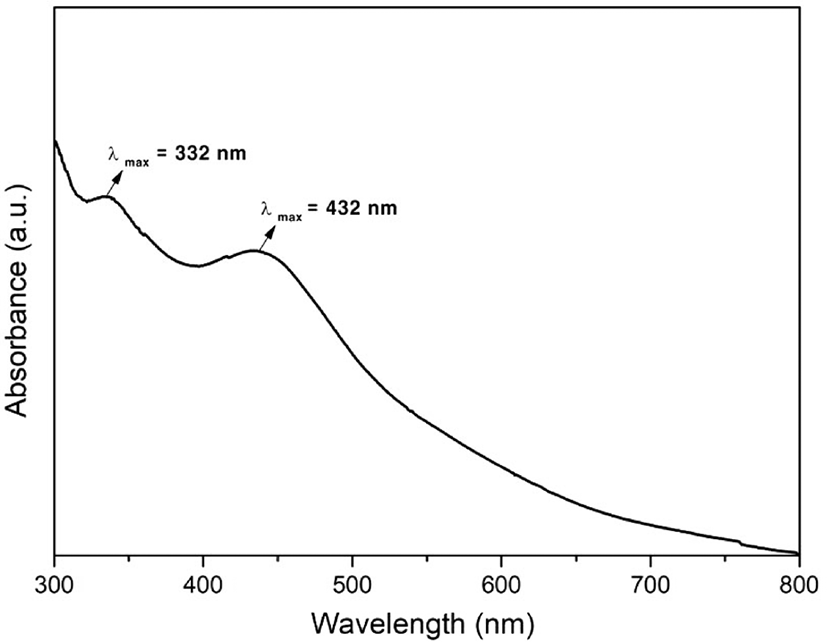
UV-vis spectroscopy was used to investigate the optical properties of fullerene oxide[C60(O)n, (n≥1)] – silver nanoparticle composites. The absorption spectra of the catalyst was recorded in the range from 200 to 800 nm. The peaks related to fullerene oxide[C60(O)n, (n≥1)] and Ag NPs were observed. As it is known, the absorption band corresponding to the Ag NPs was observed at 380-430 nm, while that corresponding to fullerene oxide[C60(O)n, (n≥1)] was observed at 330-336 nm because of the surface plasmon vibrations of the conducting electrons.18 As shown in Figure 2, the absorption peak at 432 nm can be attributed to the Ag NPs, while that at 332 nm corresponds to the fullerene oxide[C60(O)n, (n≥1)].
The structural morphology of the fullerene oxide[C60(O)n,(n≥1)] – silver nanoparticle composites was characterized using scanning electron microscopy as shown in Figure 3.
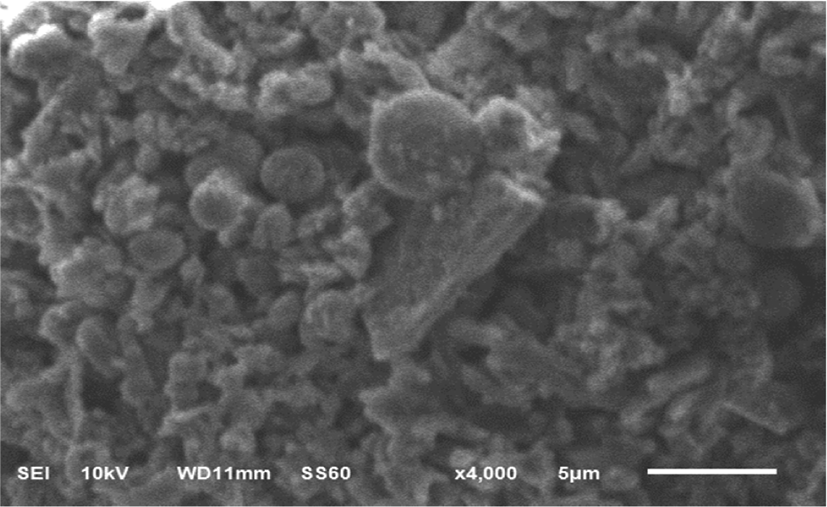
SEM images exhibited that these nanoparticle composites has two shapes of surface morphology. The fullerene oxide [C60(O)n, (n≥1)] showed multiple square shaped layers, while the Ag NPs existed as small spherical agglomerates. These results indicate that Ag NPs were successfully anchored on the fullerene oxide[C60(O)n, (n≥1)] as supporting material.
In order to investigate the catalytic activity of the hybrid nanocomposites, three isomers of nitroaniline (o-NA, m-NA and p-NA) were reduced to o-PDA, m-PDA and p-PDA, respectively, using the hybrid nanocomposites as the catalyst according to the procedures discussed in the previous sections.19,20 The effect of the Ag nanoparticles on the reduction of the nitroanilines was also investigated.19,20
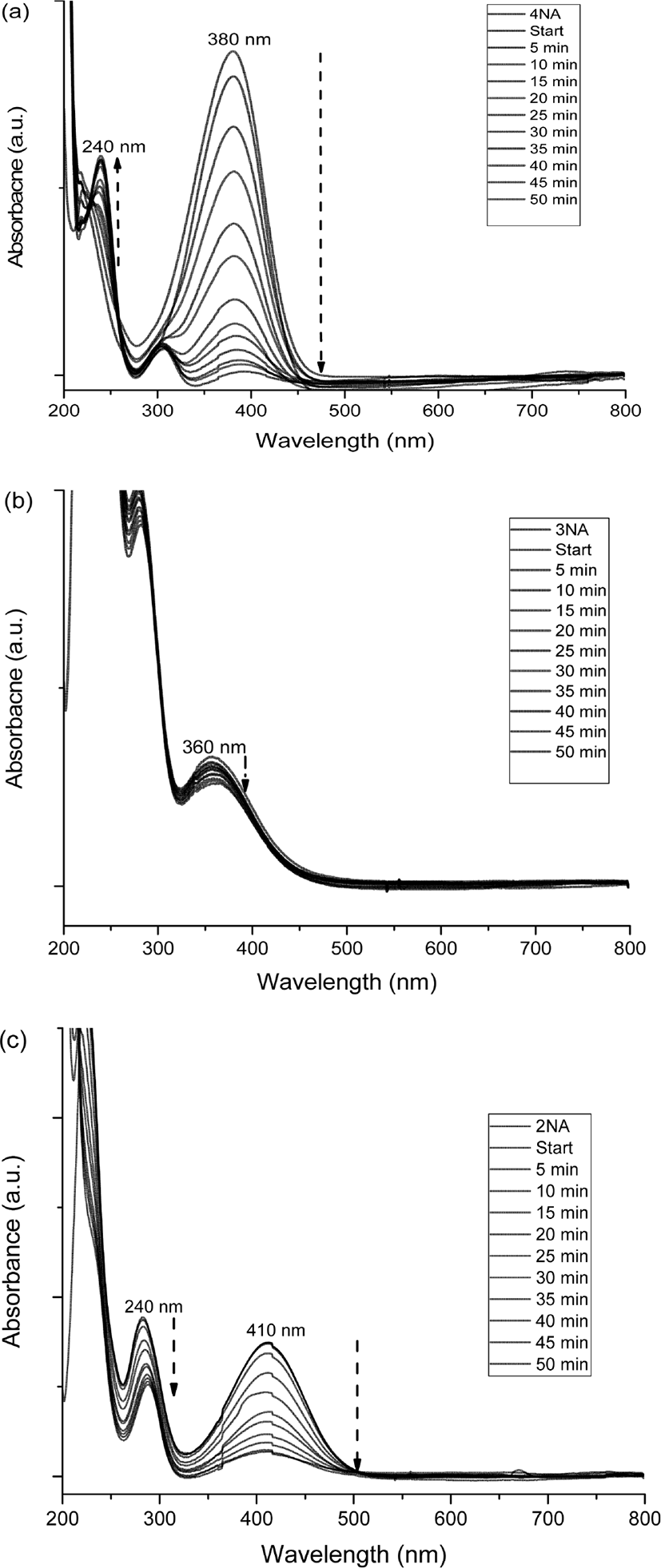
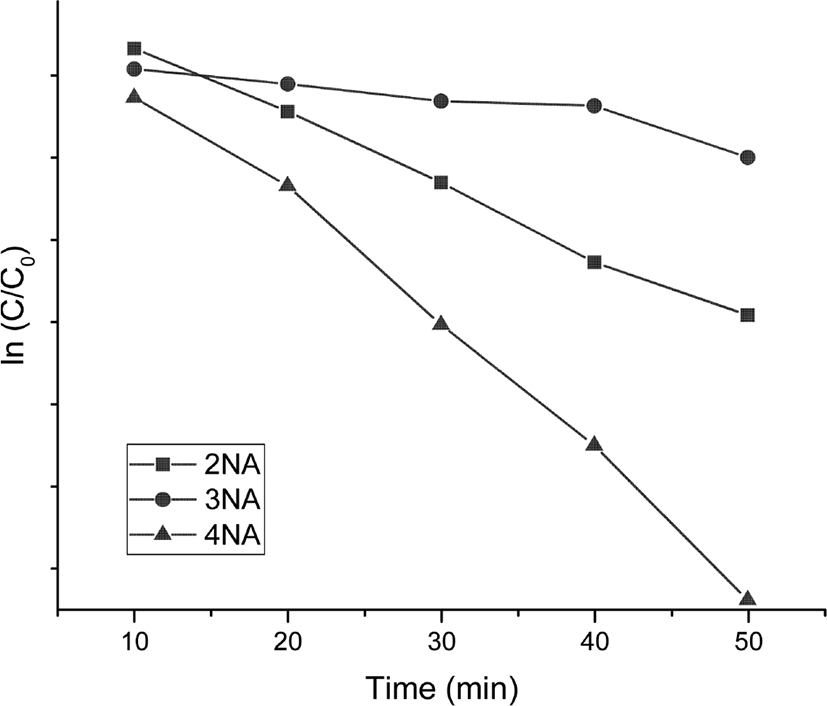
Figure 4 shows the variation of UV-vis spectra of o-NA, m-NA, and p-NA with time, which were reduced in the presence of the catalyst. The absorption peaks of o-NA to o-PDA were shown at 410 nm and 240 nm. The absorption peaks of m-NA to m-PDA were shown at 360 nm and 300 nm. The absorption peaks of p-NA to p-PDA were shown at 380 nm and 240 nm. As shown in Figure 5, the kinetics study for catalytic reduction of various nitroanilines was followed at a slope of pseudo-first order reaction rate, which is expressed by a simple equation of ln (C/C0) = −kapp · t, where the kapp is an apparent rate constant, the C/C0 is concentration ratio of different measuring state at time t and initial state. In the observation of catalytic activity, the efficiency of nitroaniline reductions has an order of 4-NA > 2-NA > 3-NA.
Conclusions
In summary, we successfully synthesized hybrid nanoparticle composites which Ag NPs were located on the surface of fullerene oxide[C60(O)n, (n≥1)] by ultrasonic irradiation. XRD, UV-vis spectrophotometer and SEM were used to characterize the hybrid nanoparticle composites which were synthesized. The hybrid nanocomposites were used as catalyst to reduce 2-,3-,4-nitroaniline to 2-,3-,4-phenylenediamine and exhibited excellent catalytic activity.






