Introduction
Recently, foldable, rollable and even stretchable flexible polymer substrates become popular. Flexible polymer sub-strates have been applied in flexible displays, flexible solar cells, flexible printed circuit boards, and touch panels.1,2 Flexible polymer substrates need to exhibit excellent optical transparency, high glass transition temperature (Tg), low coefficient of thermal expansion (CTE), and oxidation resistance.3,4 Typical transparent polymer substrates include polyethylene terephthalate,5,6 polyethylene naphthalate,7,8 and polycarbonate9,10 but their thermal stability are not as high for substrate of electronic devices..
Aromatic polyimide is a type of high-performance polymer that possesses excellent heat resistance and outstanding physical properties, making it widely used in advanced fields such as the aerospace and electronics industries.11,12 In the electronics industry, it is used in a variety of applications due to its low dielectric constant, good thermal and chemical stability. These applications include interlayer insulating films or protective films for semiconductors, multilayer films for electronic packaging, alignment layers for liquid crystal displays, and optical wave guides.13
However, typical aromatic polyimides are from deep yellow to brown in color, with low light transmittance. The reason for deep color of polyimide films is the formation of charge transfer complex (CTC) between electron donating aromatic diamines and electron accepting aromatic dianhydrides with strong absorption in the UV–Vis region.14 Aromatic polyimides are often insoluble and infusible in their fully imidized form due to their rigid chain characteristics, so they must be processed via poly(amic acid) precursors and subsequent thermal or chemical imidization.
To overcome these problems, researches have been focused on the synthesis of transparent, colorless and soluble polyimides without deterioration of their own excellent properties. These include introducing aliphatic moieties or fluorine atoms into the main chain, attaching bulky pendant groups.15-17 However, the aliphatic moieties usually cause deterioration of the inherent thermal stability of aromatic polyimides. The introduction of hexafluoroisopropylidene (6F) linkage is also known for enhancing processability and reducing color of aromatic polyimide. The 6F linkage increases the flexibility of the aromatic polyimide, breaks the conjugated structure, and hinders the formation of CTC. On the other hand, since the covalent bond between carbon and fluorine is very strong, the thermal stability of aromatic polyimide does not decrease much.18 Bulky pendant groups can not only inhibit the close-packing of polyimide chain to improve their solubility without sacrificing the thermal stability, but also reduce the formation of intermolecular CTC to form transparent and colorless films.19
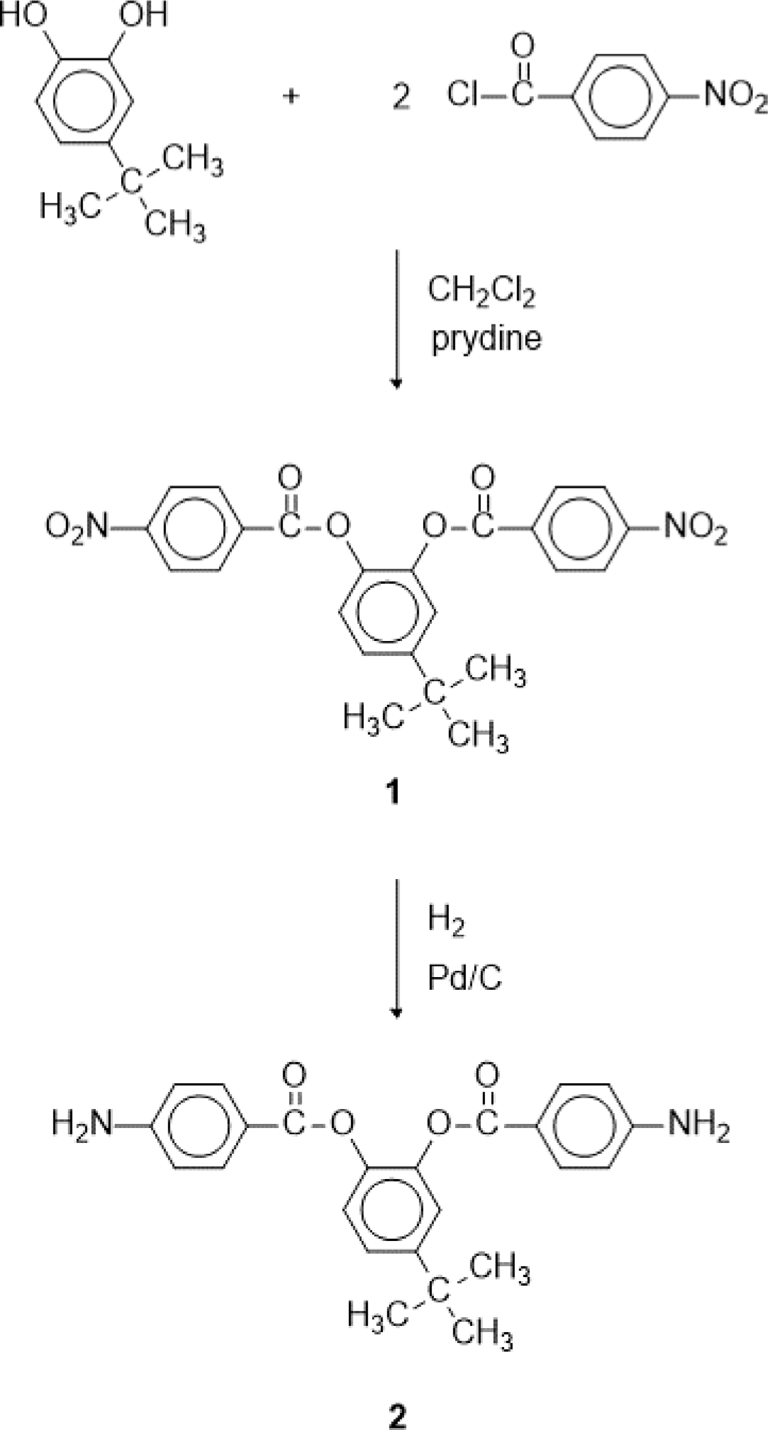
We reported a polyimide with methyl group and 6F linkage.20 The polyimide has high transparency and good thermal stability. In this study, we prepared a polyimide containing tert-butyl group. The tert-butyl group is larger than methyl group, so it is expected to improve transparency and decrease color of polyimide. An aromatic diamine was synthesized from the reaction of 4-tert-butyl catechol with 4-nitrobenzoyl chloride and subsequent reduction. An aromatic polyimide was obtained from the polymerization of the aromatic diamine with 4,4’-(hexafluoroisopropylidene) diphthalic anhydride (6FDA). The solubility, thermal stability and optical property of the aromatic polyimide were investigated.
Experimental
4-tert-Butyl catechol and 4-nitrobenzoyl chloride were used as purchased from Aldrich. 4,4’-(Hexafluoroisopropylidene) diphthalic anhydride (6FDA, Aldrich) was sublimated at 230°C. N-Methyl-2-pyrrolidinone (NMP, Aldrich), ethyl acetate (Katayama Chemical Co.), dichloromethane (Junsei Chemical Co.), pyridine (Aldrich), acetic anhydride (J.T. Baker), acetone (Aldrich), and Pd/C (Aldrich) were used as purchased.
4-tert-Butyl catechol (5.00 g, 0.03 mol), 4-nitrobenzoyl chloride (12.05 g, 0.68 mol), dichloromethane (150 mL), and pyridine (7.2 mL) were added into a 500 mL three-necked round bottom flask equipped with a stirrer and a reflux condenser. The reaction mixture was heated to 40°C and the temperature was maintained for 5 h under nitrogen. The reaction mixture was cooled to room temperature and dichloromethane was removed using a rotary evaporator. The solid product obtained was washed with 3% aqueous HCl solution, 3% aqueous KHCO3 solution, and distilled water, and dried under vacuum at 80°C for 24 h. The crude product was recrystallized from acetone to give white crystals (11.15 g, yield was 80%). The melting point was 168°C.
A suspension of 4-tert-butyl-1,2-phenylene bis(4-nitrobenzoate) (1) (5.0 g) and Pd/C (0.2 g) in ethyl acetate (100 mL) was shaken under hydrogen (50 psi) in a Parr hydrogenation apparatus at room temperature for 4 h. The Pd/C was removed by filtration and the solution was concentrated under reduced pressure to afford solid. The crude product was recrystallized from ethanol to give crystals (2) (3.27 g, yield 75%). The melting point was 216°C.
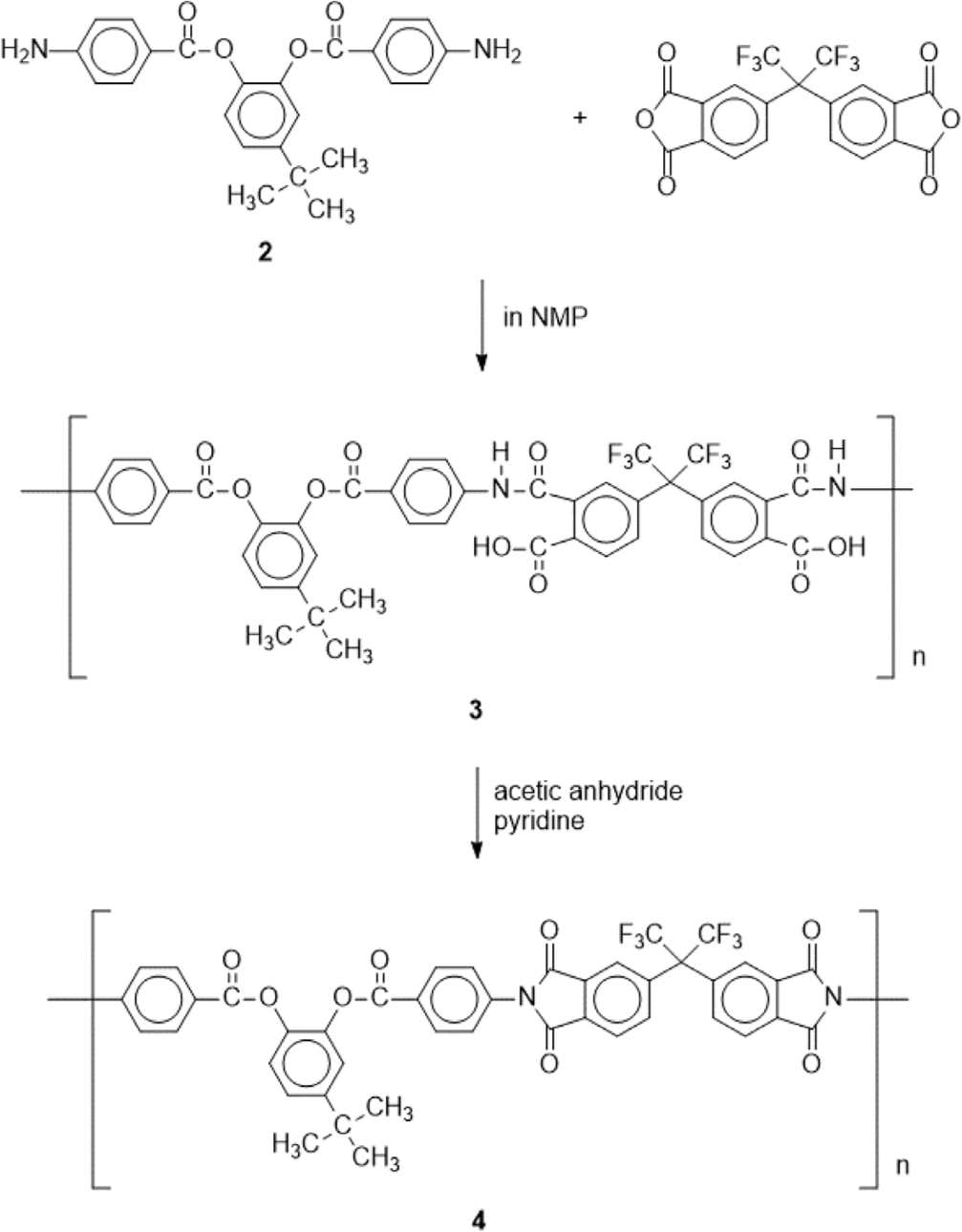
4-tert-Butyl-1,2-phenylene bis(4-aminobenzoate) (2) (0.4108 g, 1.016 mmol) and NMP (7.8 mL) were added in a 250 mL three-necked flask equipped with a stirrer and a nitrogen inlet. After 2 had been completely dissolved in NMP, 6FDA (0.4521 g, 1.018 mmol) was slowly added and the reaction mixture was stirred at room temperature for 24 h under nitrogen yielding a clear and viscous solution of poly(amic acid) (3). The poly(amic acid) (3) was chemically imidized by addition of acetic anhydride and pyridine and subsequent heating at 40°C for 24 h. The polyimide solution was cooled to room temperature and then poured into ethanol, forming a precipitate that was collected by filtration. Washing with methanol followed by drying under vacuum afforded polyimide (4) as a powder.
Melting points were measured at a heating rate of 5°C/ min on MP80 (Mettler Toledo, USA). FT-IR spectra of the compounds were obrained with a Perkin Elmer Spectrum 3 spectrophotometer. 1H NMR spectrum of the monomer dissolved in DMSO-d6 was recorded on Jeol JNM-ECP 400 Spectrometer. Thermogravimetric anaysis (TGA) was performed on TA Instruments SDT650. TGA measurement was made at a heating rate of 10°C/min in N2. Light transmittance of the polyimide film was measured using a Jasco V-770 UV-Visible Spectrophotometer.
Results and Discussion
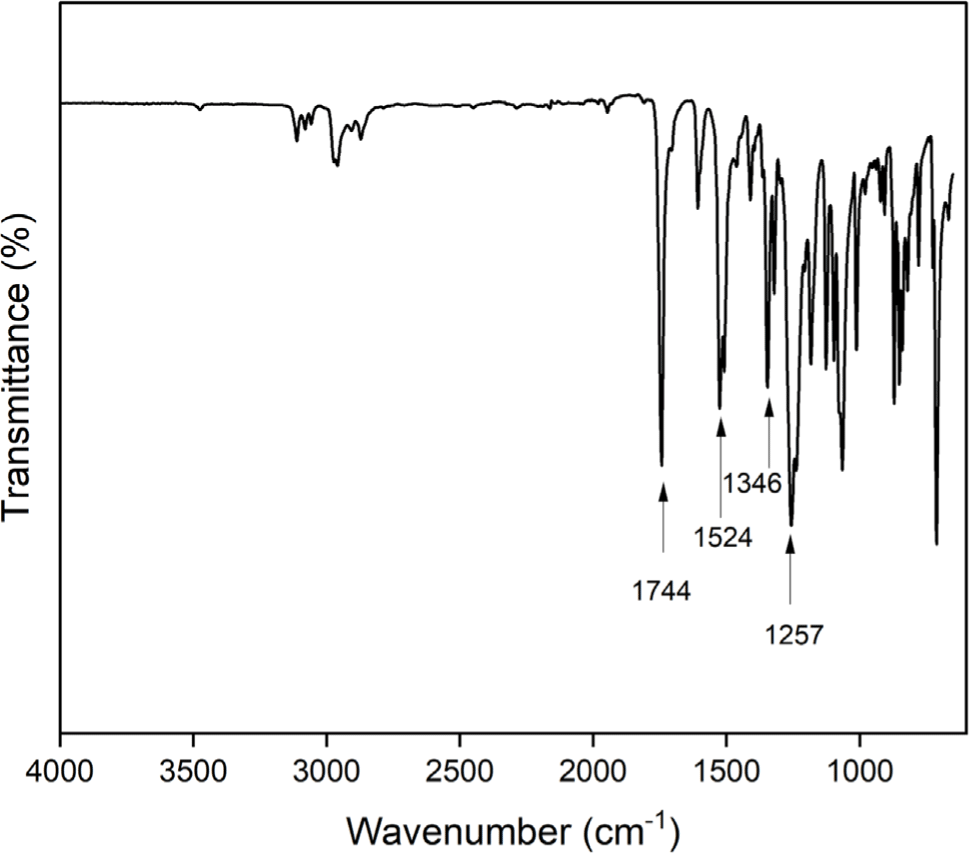
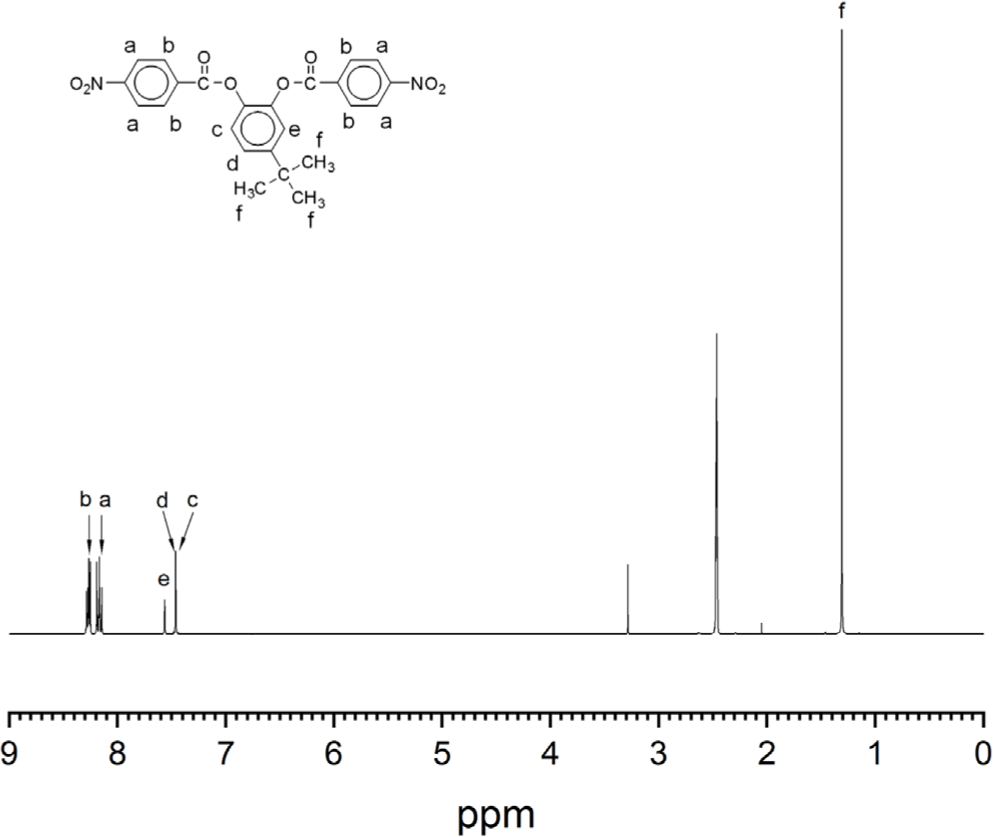
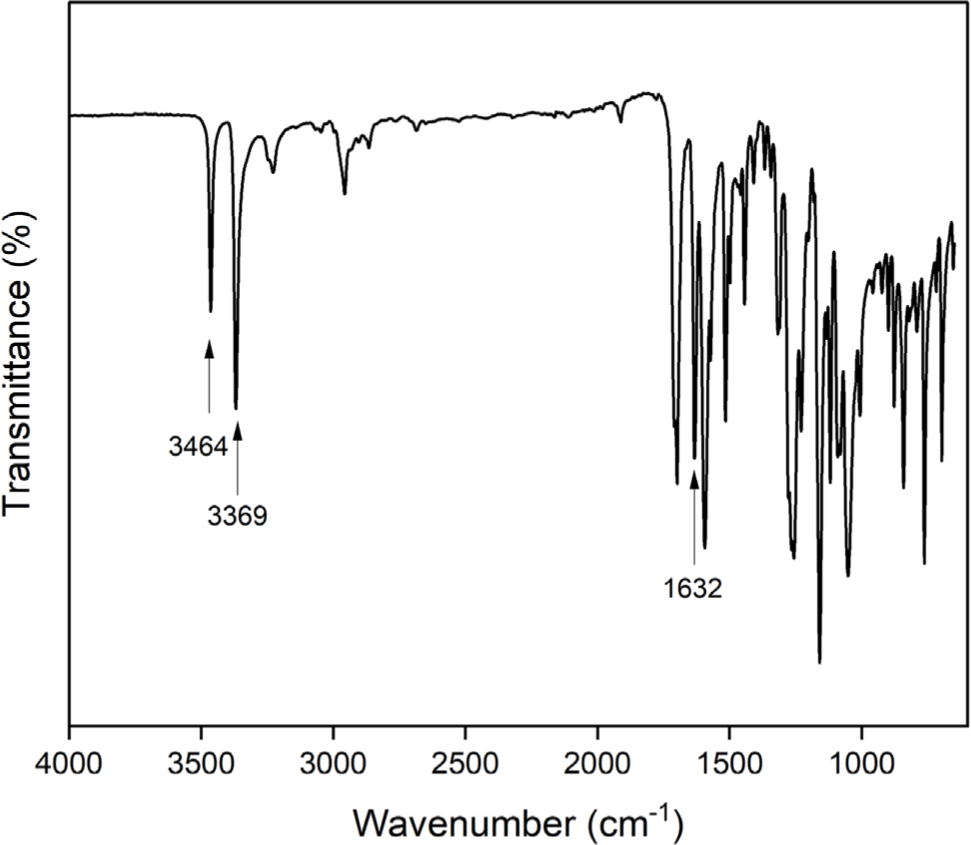
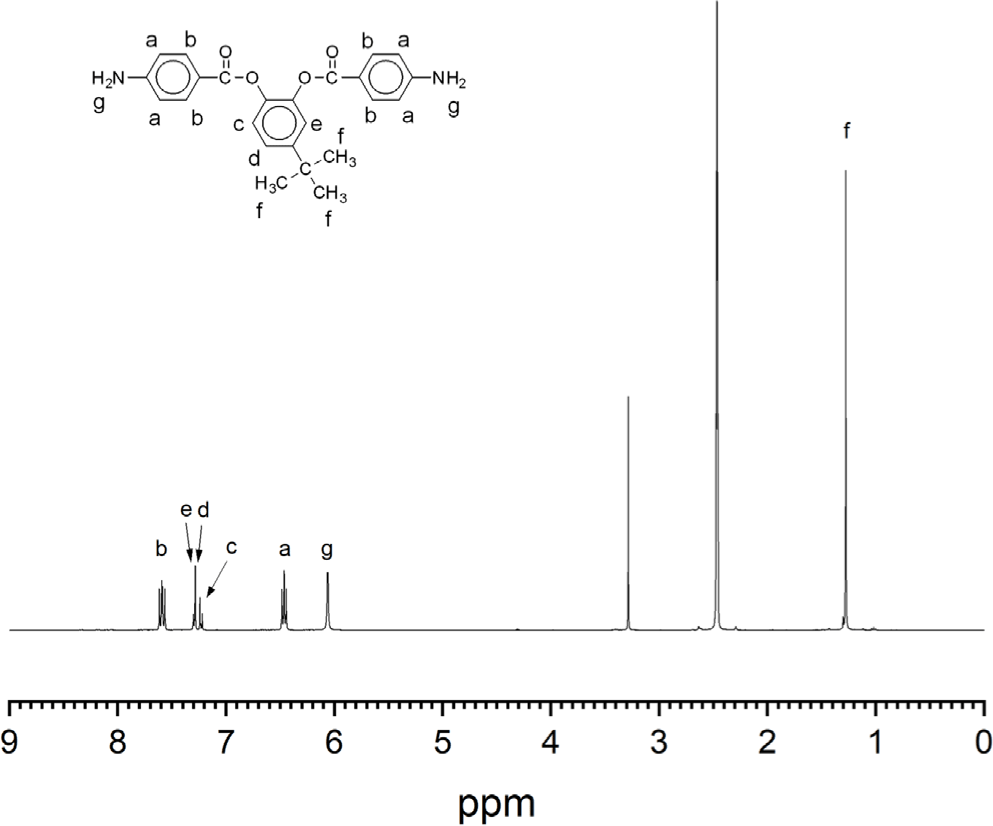
The aromatic diamine monomer containing 4-tert-butyl group, 4-tert-butyl-1,2-phenylene bis(4-aminobenzoate) (2) was prepared according to the reaction sequence of Scheme 1. The 4-tert-butyl-1,2-phenylene bis(4-nitrobenzoate) (1) was synthesized from 4-tert-butyl catechol and 4-nitrobenzoyl chloride in high yield. The dinitro compound 1 was reduced to corresponding diamine monomer 2 using catalytic hydrogenation over Pd/C catalyst in a Parr shaking apparatus. The structure of dinitro compound 1 and diamine monomer 2 was confirmed by FI-IR and 1H NMR spectroscopy. Figure 1 is FT-IR spectrum of dinitro compound 1. The FT-IR spectrum shows absorption bands at 1744 cm-1 according to the C=O asymmetric stretching, 1257 cm-1 to the C-O stretching, 1524 cm-1 to the N-O asymmetric stretching, and 1346 cm-1 to the N-O symmetric stretching. Figure 2 is the 1H NMR spectrum of dinitro compound 1. Aromatic proton peaks are found in the range from 7.4 to 8.3 ppm, while the peak at 1.3 ppm is assigned to tert-butyl groups peaks at 1.3 ppm were observed. Figure 3 is FT-IR spectrum of diamine monomer 2. After reduction, the characteristic absorptions of nitro group are disappeared and the characteristic absorptions of amino group at 3464 and 3369 (N-H stretching) and 1632 cm-1 (N-H bending) are observed. Figure 4 is the 1H NMR spectrum of diamine monomer 2. Aromatic proton peaks are found in the range from 6.4 to 7.7 ppm, while amine proton peak at 6.1 ppm and tert-butyl group peak at 1.3 ppm are observed. The integral value of NMR spectrum matched the number of protons in diamine monomer 2.
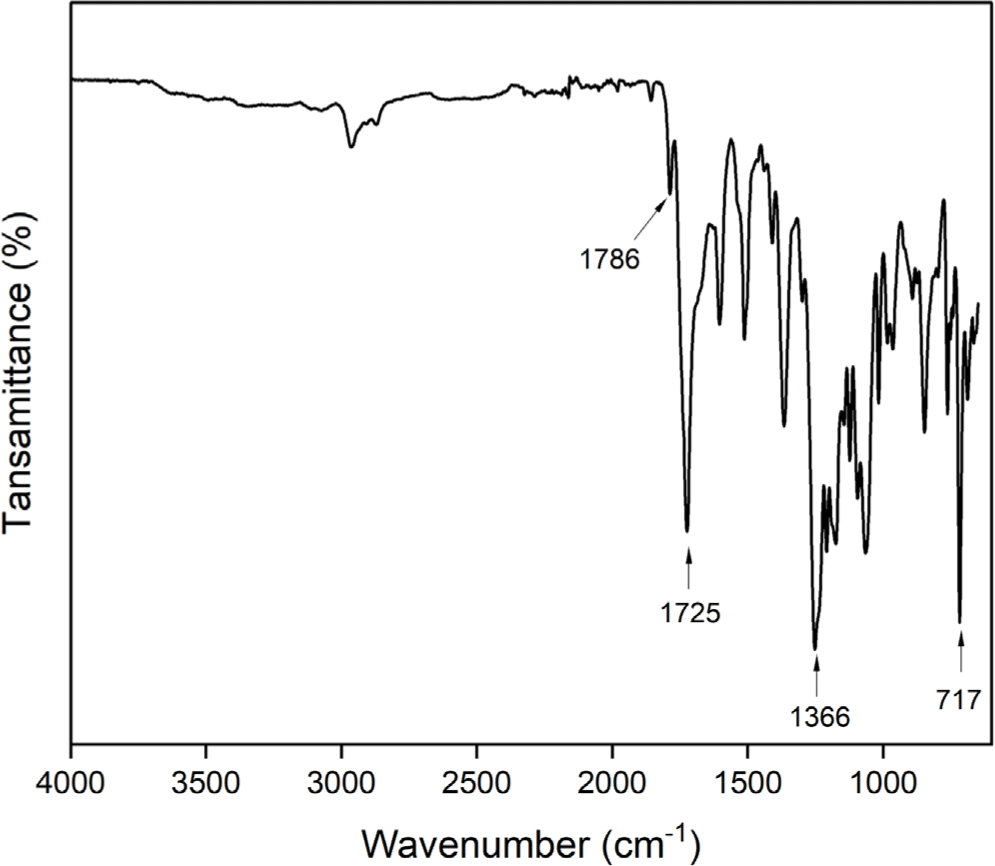
Polyimide was prepared by the conventional two step polymerization method starting from 4-tert-butyl-1,2-phenylene bis(4-aminobenzoate) (2) and 4,4’-(hexafluoroisopropylidene) diphthalic anhydride (6FDA) through ring opening reaction forming poly(amic acid) and subsequent thermal or chemical cyclodehydration (Scheme 2). The poly(amic acid) (3) was prepared by reacting stoichiometric amount of 4-tert-butyl-1,2-phenylene bis(4-aminobenzoate) (2) with 6FDA at a concentration of 15 wt% solid in NMP. The ring opening addition reaction at room temperature for 24 h yielded poly(amic acid) solution. After dilution of the solution to 7 wt%, subsequent chemical cyclodehydration with a mixture of pyridine and acetic anhydride gave the fully imidized polyimide (4). The structure of the polyimide 4 was confirmed by FT-IR spectroscopy as shown in Figure 5. The absorption bands at 1786 (C=O asymmetric stretching), 1725 (C=O symmetric stretching), 1366 (C-N stretching), and 717 cm-1 (C=O bending) corresponding to the characteristic imide bands are observed.
Aromatic polyimides generally have a color because of charge-transfer complex (CTC). When an electron donating diamine moieties of aromatic polyimide are close to an electron accepting dianhydride moieties, CTC forms and induces optical absorption at wavelengths in the visible range. There are several methods to decrease the color of aromatic polyimide. One of them is to modify polyimides with electronegative components such as fluorinated groups which reduce the electron density in the polymer chains and suppress CTC formation. The other method is to incorporate bulky pendant group that introduce steric hindrance to interchain CTC formation. However, the bulky pendant group also affects the macromolecular packing, and thus deteriorates the thermal stability and mechanical strength.
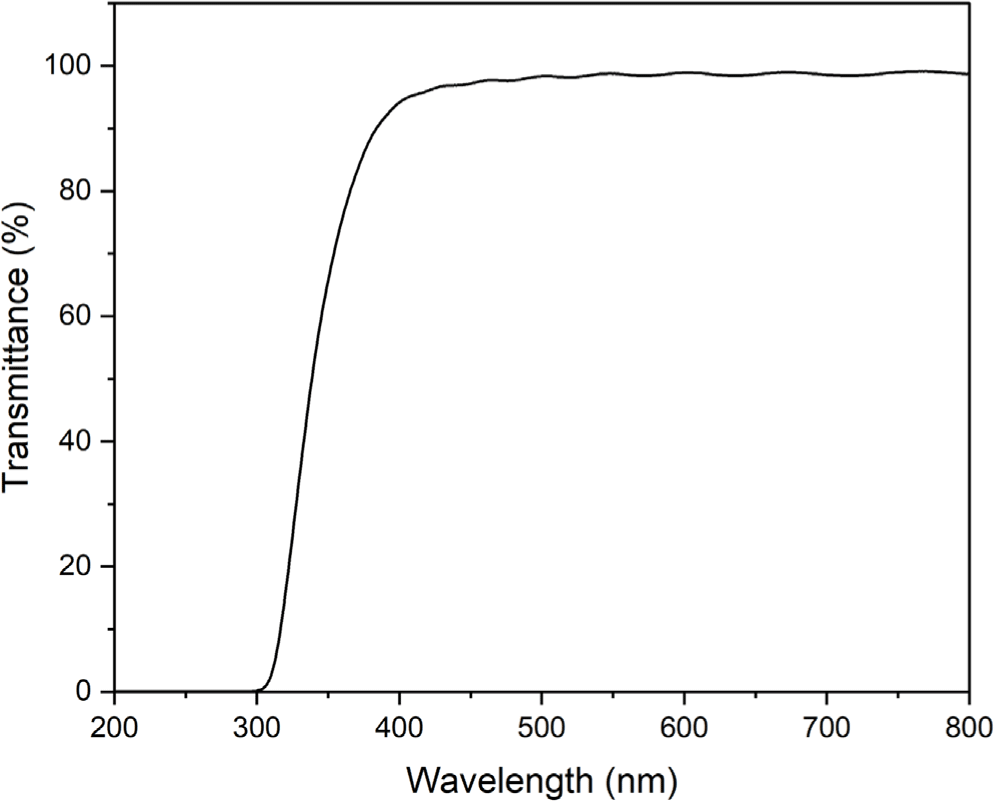
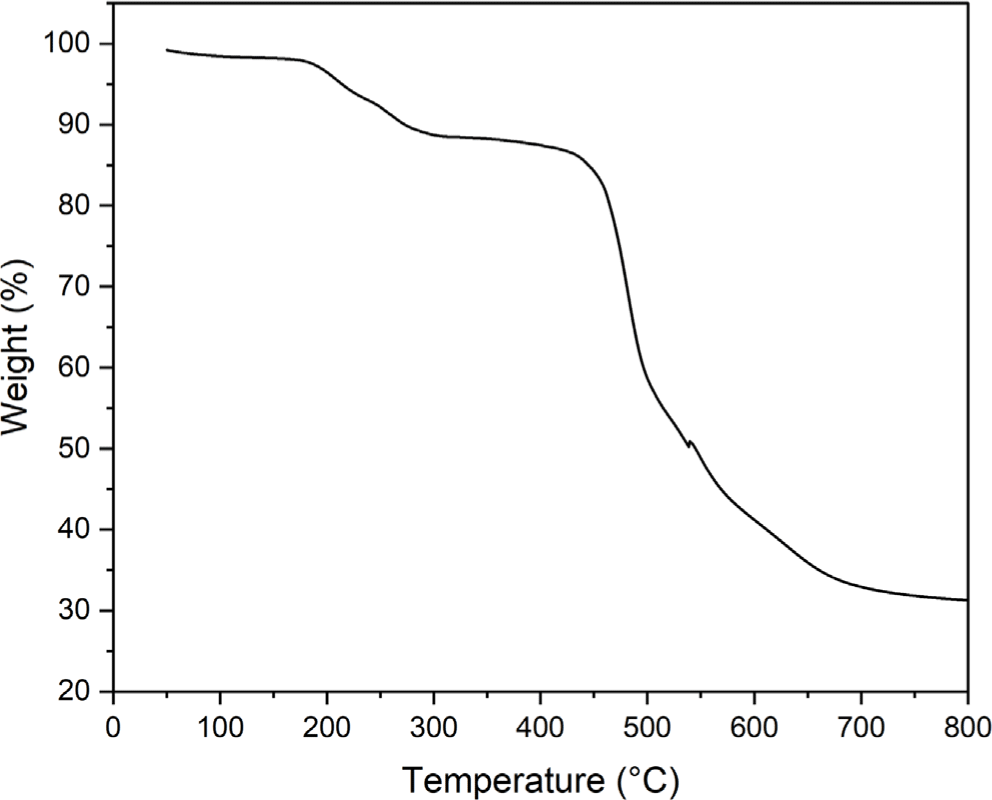
The film prepared by casting of the polyimide 4 was transparent and colorless. Electron-withdrawing hexafluoro-isopropylidene (6F) linkage in dianhydride moieties hinder the movement of π electrons and tert-butyl group in diamine moieties increases the distance between the main chain of polyimide 4, resulting in a colorless polyimide. Figure 6 shows the light transmittance measurement results of a film of the polyimide 4. The polyimide 4 shows a high transmittance above 90% in a wavelength range of 400-700 nm. The thermal stability of the synthesized polyimide 4 was evaluated by TGA (Figure 7). The polyimide 4 shows a two-step thermal degradation. In the temperature range of 180-300°C, entrapped NMP is released whereas the real degradation of the polyimide 4 begins at 400°C. It was confirmed that weight loss occurred at a lower temperature due to the effect of 6F linkage and pendant tert-butyl group.
Conclusions
An aromatic diamine containing pendant tert-butyl group was synthesized by reacting 4-tert-butyl catechol with 4-nitrobenoyl chloride and subsequent reduction. The chemical structure of the obtained 4-tert-butyl-1,2-phenylene bis(4-aminobenzoate) was confirmed by FT-IR and 1H NMR. A polyimide was prepared by reacting 4-tert-butyl-1,2-phenylene bis(4-aminobenzoate) with 6FDA, and chemical imidization. The obtained polyimide was dissolved in NMP and casted as a flexible and colorless film. As a result of TGA analysis, 6F linkage and pendant tert-butyl group lowered the thermal stability of the polyimide.