Introduction
Heat management is a critical issue in high power electronics and mobility applications. The excess heat not only damages the devices but also reduces their service life. Most components used in semiconductor devices face performance degradation due to accumulated heat. Therefore, the development of heat dissipation material materials and structural design is crucial. Various types of thermal conductive materials in the form of pad, grease, and gel have been commercialized. Recently thermally conductive composites, which generally consist of a polymer matrix and ceramic filler to transfer heat but insulate electricity, are a major research area.1-5 The thermal conductivity is influenced by many factors such as the intrinsic thermal conductivity of the polymer matrix and the fillers, and their interfaces. High thermal conductivity can be achieved simply by increasing the amount of ceramic filler, but it causes poor processability and deterioration of mechanical properties of the products. More heat transfer channels are formed through the contact of fillers, resulting in higher thermal conductivity. Several attempts have been made to increase thermal conductivity while minimizing filler content.6-8
The thermal resistances at the filler-matrix interfaces and the filler-filler interfaces significantly affect the thermal conduction performance. When nanoparticles are used, they can easily create a continuous heat transfer pathway within the matrix even at low loading.9-12 However, the thermal conductivity of nanocomposites is typically lower than the composites filled with conventional micro-sized fillers. This is primarily due to the challenges posed by phonon scattering at the polymer-particle interface and non-uniform dispersion of the fillers. To improve thermal conduction across the interfaces while minimizing phonon boundary scattering, various surface functionalization treatments have been applied to nano-sized fillers.13-15 Boron nitride (BN) is regarded as an ideal thermally conductive filler for polymer composites because of its high in-plane thermal conductivity.16-20 However, similarly to other polymer composites, the significant interfacial thermal resistance at the filler-matrix and the filler-filler interfaces hinders heat conduction. Therefore, the construction of an effective heat conduction pathway is crucial for the development of polymer composites. This pathway helps create a three-dimensional (3D) interconnected network for heat transport and ensures good filler distribution, which is essential for maintaining mechanical property.
In this study, three different carboxylic acids having different chain length, i.e., succinic acid (SUA), adipic acid (ADA), sebacic acid (SA), were introduced onto nano-sized boron nitride (BN) to improve the compatibility with epoxy/ benzoxazine resin. We measured various physical properties including viscosity, storage modulus, tan δ, and adhesion strength have been measured to examine the influence of carboxylic acid chain length. Additionally, we studied the thermal conductivity of the adhesive with incorporation of untreated (N-BN) and treated BN (SUA-BN, ADA-BN, SA-BN).
Experimental
A low-viscosity cycloaliphatic epoxy resin (Celloxide 2021P, Daicel Corporation) was blended with benzoxazine (P-d type, Shikoku Chemicals, Kagawa, Japan) in a weight ratio of 30/70 to serve as the matrix. The mixture was thermally cured with the addition of 30 wt% of curing agent, i.e., 4-methylhexahydrophthalic anhydride (RIKACID MH, Japan Chemical), and 3 wt% of accelerator (2MA-OK, Shikoku Chemicals). Nano-sized boron nitride (BN) with an average particle size of 5 μm was purchased from Denka Co. Ltd. Three different carboxylic acids were used to modify the BN nanoparticles: succinic acid (SUA, MW = 118.09 g/ mol, Sigma-Aldrich), adipic acid (ADA, MW = 146.14 g/ mol, Sigma-Aldrich), sebacic acid (SA, MW = 202.25 g/mol, Millipore). For surface modification, 1.0 g of each carboxylic acid was dissolved in 300 mL of ethanol, followed by the stepwise addition of 100 g of BN. This homogeneous mixture was heated to 60°C for 4 hours and washed several times with deionized water. The surface-modified BN was then dried at 90°C for 12 hours. The BN powder was evenly dispersed in the epoxy/benzoxazine mixture using a three-roll mill (EXAKT 80E, EXAKT Technologies, Germany). Thermal curing of the final mixture was conducted at 175°C for 60 minutes.
The thermal stability of untreated and surface-modified boron nitride (BN) was analyzed using thermogravimetric analysis (TGA, Model Pyris 1, Perkin Elmer, USA). Approximately 5 mg of BN powder was placed in an aluminum pan and heated at a rate of 10°C/min from 30°C to 500°C in an air atmosphere. Mechanical characteristics of epoxy/ benzoxazine/BN adhesives were monitored using dynamic mechanical analysis (DMA, Seiko Exstar 6000, DMA/ SS6100, SEICO). The specimen having a rectangular shape (20 mm in length × 6 mm in width × 0.3 mm in thickness) was heated at 5°C/min from 20°C to 280°C. The test was conducted for the 30% strain at a fixed frequency of 1 Hz. The viscosity of the mixtures was measured at various spindle rotation speeds ranging from 0.1 rpm to 5 rpm using a Brookfield rotational viscometer (DV2T, Brookfield Engineering Labs). A die shear test was conducted using a Dage Series 4000 (USA) to measure adhesion strength. The adhesive was applied to three metallic lead frames, i.e., silver (Ag), and copper (Cu), gold (Au), and a square-shaped silicon die (1.25 mm × 1.25 mm, 350 μm thick) was attached. After thermal curing, the force required to separate from the die to the lead frame was measured. Thermal conductivity was measured using the laser flash method (LFA447, Netzsch Instruments). Thermal conductivity was calculated from the thermal diffusivity obtained using a square sheet with 10 mm in length and 1 mm in thickness.
Results and Discussion
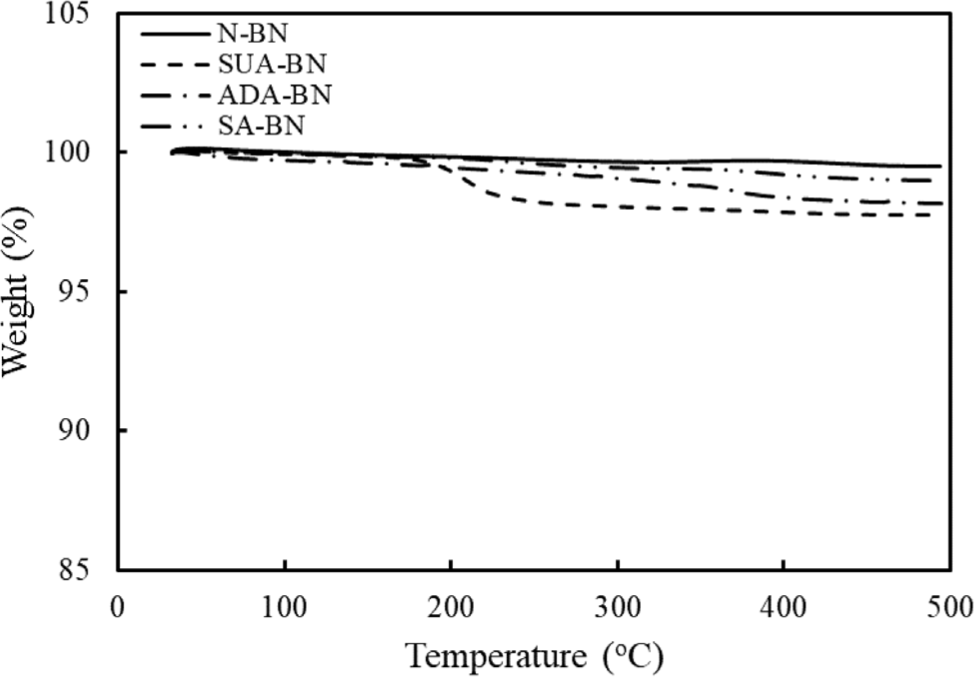
Figure 1 displays the TGA curves of the untreated and surface modified boron nitride (BN) nanoparticles. Pure BN (referred to as N-BN) is thermally stable up to 500°C. In contrast, the surface-modified BNs, which include SUA-BN, ADA-BN, and SA-BN, exhibit varying weight loss behaviors. The SA-BN shows a gradual weight loss of around 1 wt% at 500°C. However, weight loss occurs at approximately 200°C for SUA-BN and at 300°C for ADA-BN. This can be attributed to the boiling points of respective carboxylic acid, i.e., 294°C for sebacic acid (SA), 265°C for adipic acid (ADA), 235°C for succinic acid (SUA). The weight loss is closely related to the volatilization of the carboxylic acid coating layer. Since the carboxylic acid is physically bonded to the BN surface, it vaporizes readily near its boiling point. The removal of this surface layer can create defects at the interface between the matrix resin and the filler, resulting in a decrease in mechanical properties.
Viscosity is a crucial criterion in determining industrial applicability of adhesives. Although the bond line thickness is easier to handle at high viscosity, low viscosity is preferable for achieving a uniform thin coating over a larger area. When an adhesive is used as a thermal interface material (TIM) in semiconductor processes, low viscosity is advantageous for forming a consistent film and minimizing layer thickness. The viscosity of an epoxy/benzoxazine adhesive containing 30 wt% BN is summarized in Table 1. The viscosity of the adhesive with untreated BN (N-BN) ranges from 82,850 cps at 0.1 rpm to 63,460 cPs at 5 rpm, depending on the rotation speed. However, when the same amount of surface-modified BN is added, the viscosity decreases significantly. The viscosity for the surface-modified BN types is observed as follows: 66,280 to 51,780 cPs for SUA-BN, 66,280 to 45,980 cPs for ADA-BN, and 70,420 to 52,110 cPs for SA-BN. The viscosity of the adhesive is closely related to the interfacial wettability between the resin and BN, suggesting that enhanced compatibility may contribute to the decrease in viscosity. A shear thinning phenomenon, where viscosity decreases with increasing shear speed (rpm), is observed for all the samples. The thixotropic index (TI), defined as the ratio of viscosities measured at 0.5 rpm and 5 rpm, is found to be between 1.2 and 1.3.
When the BN is added to an epoxy/benzoxazine matrix, an increase in storage modulus is expected to increase due to the intrinsic high modulus of ceramic fillers. Table 2 provides the storage modulus values for composites. The storage modulus of the adhesives containing 30 wt% untreated BN (N-BN) is measured at 12 GPa at 25°C. This value decreases abruptly to 9.2 GPa at 150°C and further drops to 0.09 GPa at 250°C. Similarly, with the addition of surface-treated BN at the same concentration, comparable modulus values are observed across the investigated temperature range. Notably, the tan δ peak of the composite increases slightly above 5°C observed in a range of 197~200°C when surface modification is applied. A High tan δ value indicates improved interfacial interaction and dispersibility between the epoxy/benzoxazine resin and the BN nanoparticles. Additionally, the shore D hardness of the composites containing untreated and treated BN ranges from 83 to 86.
Figure 2 illustrates the die shear strength of the epoxy/ benzoxazine/BN adhesives when applied to various metallic lead frames, specifically silver (Ag), copper (Cu), and gold (Au). The tests were also conducted at elevated temperatures of 180 and 250°C, which simulate the wire bonding and reflow environments encountered in the semiconductor packaging process. At 25°C, the adhesive containing untreated BN (N-BN) exhibits a shear strength of 5.8 kgf/cm2 for Ag, 6.7 kgf/cm2 for Cu, and 6.8 kgf/cm2 for Au. While surface treatments generally improve adhesion strength, the degree of the enhancement varies depending on the type of carboxylic acid used. For silver (Ag), the adhesion strength of SUA-BN is nearly twice that of N-BN, followed by ADA-BN and SA-BN. In contrast, the increase in adhesion strength for Cu and Au is not as pronounced although a higher strength value is still noticed for SUA-BN. Intrinsic nature of lead frame matals, i.e., oxidation of Cu and inertness of Au, may affect the adhesion strength. It is important to highlight that the order of adhesion strength remains consistent, with SUA-BN > ADA-BN > SA-BN across all metal types. This suggests that carboxylic acids with shorter chain length are more effective in enhancing adhesion. Higher shear strength is attributed to a promoted reaction between carboxylic acids and the epoxy/benzoxazine resin, as well as improved dispersion of BN nanoparticles, regardless of the type of metallic lead frame used.
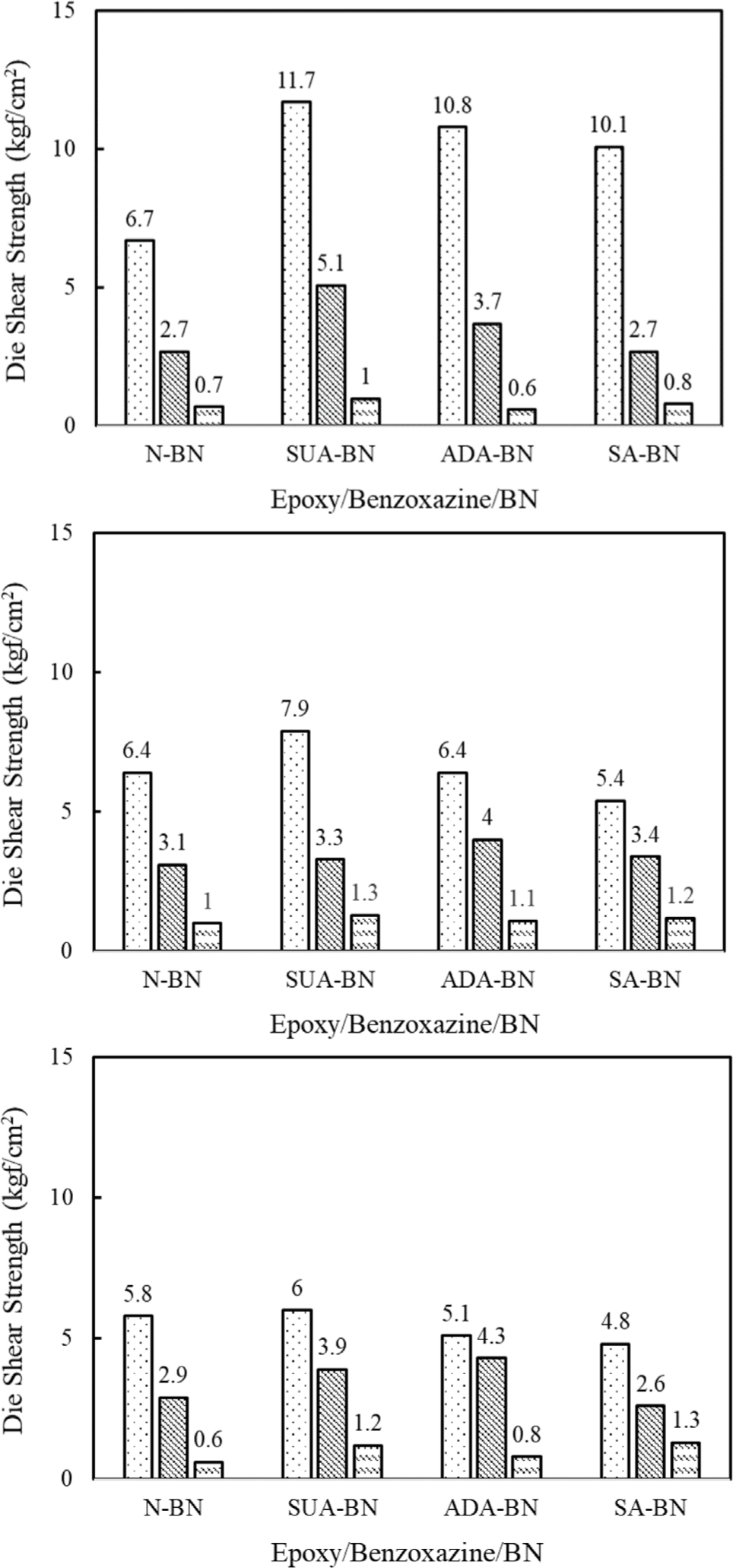
The adhesion strength of both untreated and treated BN decreases significantly as the test temperature increases. The temperature-dependent storage modulus confirms that the epoxy/benzoxazine/BN compounds soften when heated above 150°C, which contributes to a reduction in adhesion strength. Additionally, a greater mismatch in thermal expansion coefficients between the resin and the substrate can further lower adhesion strength. At 180°C, the adhesion strength of SUA-BN decreases by 56% for Ag, 58% for Cu, 35% for Au, respectively. At 250°C, adhesion strength is further reduced to nearly 1 kgf/cm2 for all adhesives.
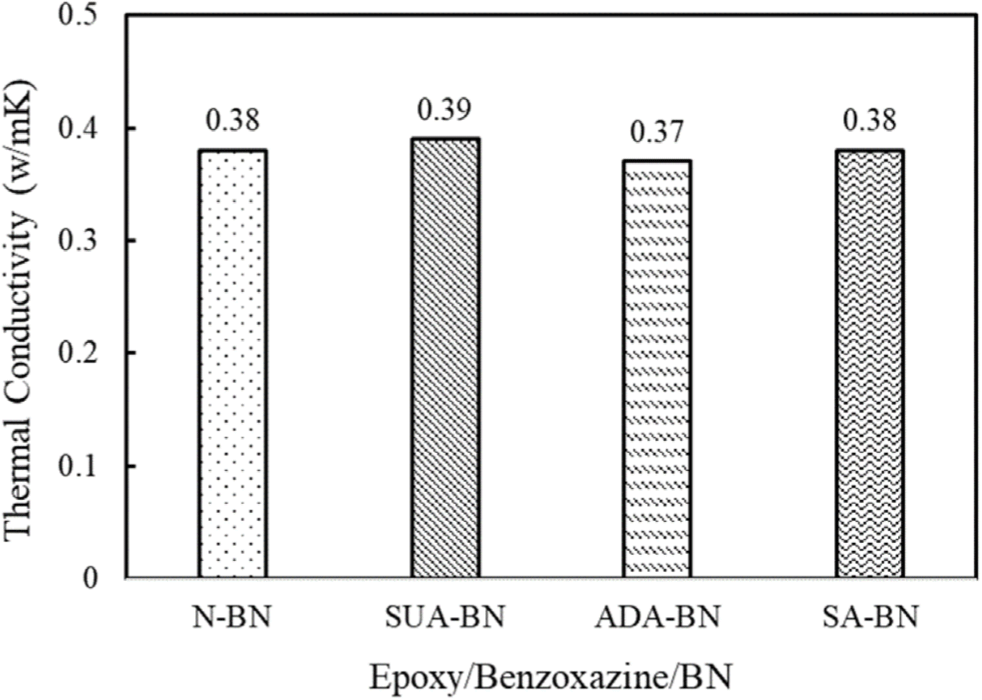
The thermal conductivity of the adhesives filled with 30 wt% both untreated and treated BN is illustrated in Figure 3. The adhesives containing untreated BN (N-BN) shows a thermal conductivity of 0.38 W/mK. Despite the addition of nano-sized BN, noticeable change in thermal conductivity value is not observed because the BN particles remain in a dispersed state within the polymer matrix, leading to insufficient thermal conductive pathways. When surface-modified BN is used instead of N-BN, the adhesives display a similar thermal conductivity ranging from 0.37 to 0.39 W/ mK, suggesting that surface modification has a negligible impact on thermal conductivity. At lower filler concentrations, the interface between the matrix and the filler plays a more significant role in determining thermal conductivity than the filler-filler interface. The positive effects on thermal conductivity by the suppression of phonon scattering and void formation at the interface and may be compensated by the lowered thermal conductivity of BN with the introduction of carboxylic acid layer.
Conclusions
The experimental study demonstrated the effects of surface modification of nano-sized boron nitride (BN) particles on mechanical properties and thermal conductivity of epoxy/ benzoxazine adhesives. The introduction of carboxylic acid resulted in reduced viscosity of the adhesives, indicating improved dispersibility and processability. Additionally, a higher tan δ peak suggested enhanced interactions between the matrix resin and BN fillers. At the same BN content, a significant increase in adhesion strength was noted, particularly for the silver (Ag) lead frame tested at temperatures of 25°C, 180°C, and 250°C. A carboxylic acid layer with a shorter chain length was found to be particularly beneficial in enhancing adhesion strength. However, this study found that there was no noticeable improvement in thermal conductivity through surface modification. It is worth noting that the structure of BN effectively increases in-plane thermal conductivity.






