Article
Synthesis of Gold Nanoparticles Using Fullerene Oxide and Their Catalytic Activity for Reduction of 4-Nitroaniline
Geun Wook Park*, Jeong Won Ko**, Weon Bae Ko*,**,***,†
*Department of Convergence Science, Graduate School, Sahmyook University, 815 Hwarang-ro, Nowon-gu, Seoul 01795, Republic of Korea
**Nanomaterials Research Institute, Sahmyook University, 815 Hwarang-ro, Nowon-gu, Seoul 01795, Republic of Korea
***Department of Chemistry, Sahmyook University, 815 Hwarang-ro, Nowon-gu, Seoul 01795, Republic of Korea
© Copyright 2019 The Rubber Society of Korea. This is an Open-Access article distributed under the terms of the
Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits
unrestricted non-commercial use, distribution, and reproduction in any
medium, provided the original work is properly cited.
Received: Apr 04, 2019; Revised: Apr 29, 2019; Accepted: May 02, 2019
Published Online: Jul 31, 2019
ABSTRACT
Gold nanoparticles were synthesized by reacting potassium tetrachloroaurate (KAuCl4), potassium carbonate (K2CO3), and isopropyl alcohol in the presence of fullerene oxide [C60(O)n, n ≥ 1], which was, in turn, prepared from [C60] fullerene and m-chloroperoxybenzoic acid under refluxing conditions. The crystallinity and morphology of the prepared gold nanoparticles were confirmed by UV-vis spectroscopy, X-ray diffraction, and scanning electron microscopy. The activity of the gold nanoparticles in the reduction of 4-nitroaniline was measured in order to determine its capability as a catalyst.
Keywords: fullerene oxide; gold nanoparticles; UV-vis spectroscopy; catalyst; reduction of 4-nitroaniline
Introduction
After Kroto et al. discovered [C60] fullerenes in 1985, a number of studies were conducted on a variety of possible applications.1 Owing to its semiconductor properties and ability to absorb electrons, the use of fullerene as an oxidizing agent has been extensively investigated.2 Furthermore, modifications of the surface or insertion of other molecules inside the empty space of fullerene have also been explored in order to tune its physical and chemical properties.3 The oxidized fullerene has different types of functionalities and needs to be studied for its effects.4 In particular, many researcher are interested in the ability of oxidized fullerene, efficiently converting metal ions into metallic nanoparticles. Recently, nanosized metal particles have received much interest due to their potential applications in microelectronics, photocatalysis, magnetic devices, chemisorption, aerosols, and powder metallurgy.5 Nanoscale metal particles can produce highefficiency catalysts since their surface area is larger than the bulk metal size. The synthesis of nanoparticles of the desired size and shape is of tremendous significance in the emerging field of nano-technology.6 Especially, gold nanoparticles have different properties depending on their shape and size.7-12 The size and shape dependent physical properties of inorganic nanoparticles provide tunable materials with broad potential applications.13 Generally, the size of the metal nanoparticles, the shape, and the surface variation are some of the most important factors that may affect their physical/chemical properties.14 For instance, gold nanoparticles can absorb and scatter light stronger depend on size and shape than other metal nanoparticles.15 Many of these properties, including strong absorption, scattering and intense local field generation result from the coherent oscillation of conduction band electrons, known as localized surface plasmon resonance.16 In general, gold nanoparticles have higher chemical stability than silver nanoparticles when they are dispersed in aqueous solutions and biological media.17,18The higher chemical stability has led to abundant applications in the fields of spectroscopy, catalysis, energy, and biology.19-21 These features will be used to reduce 4-nitroaniline to 1,4-phenylenediamine. In laboratories and industries, various types of indicators and organic dyes are used for various purposes.22 When such substances are disposed of without a proper treatment, they can seriously pollute the ecosystem including household water supply. In particular, 4-nitroaniline is harmful to all aquatic organisms and releasing 4-nitroaniline can cause long-term damage to the environment.23 On the contrary, the catalytic reduction of 4-nitroaniline allows it to be converted into a less toxic compound like 1,4-phenylenediamine in an aqueous medium.24 Hence, it is desirable to develop an environmental friendly method to achieve the reduction of 4-nitroaniline in an aqueous medium.25
Experimental
1. Materials and equipment
Potassium tetrachloroaurate (Sigma-Aldrich), potassium carbonate (Duksan), fullerene [C60] (TCI), m-chloroperoxybenzoic acid (Sigma-Aldrich), isopropyl alcohol (Samchun), and 4-nitroaniline (Sigma-Aldrich) were used in this work. The gold nanoparticles were identified by using an ultraviolet-visible (UV-vis) spectrophotometer (Shimadazu, UV-1601 PC), scanning electron microscope (SEM, JEOL Ltd, JSM-6510) and X-ray diffractometer (Bruker, D8 Advance).
2. Synthesis of fullerene oxide [C60(O)n, n ≥ 1]
The mixture of [C60]fullerene (20 mg) and m-chloroper-oxybenzoic acid (96 mg) dissolved in toluene (60 mL) was refluxed for 5 h. After extracting all the volatiles, the remaining solid was washed with methyl alcohol to remove the excess of m-chloroperoxybenzoic acid. Then, the produced solid substance was dried in an oven and fullerene oxide [C60(O)n, n ≥ 1] was obtained as a dark-brown solid.26
3. Preparation of gold nanoparticles
Potassium carbonate (K2CO3, 300 mg) and potassium tetrachloroaurate (KAuCl4, 40 mg) were dissolved in 250 and 100 mL of distilled water, respectively. The potassium carbonate solution (10 mL) and fullerene oxide [C60(O)n, n ≥ 1] (6 mg) were added to the potassium tetrachloroaurate solution, and the mixed solution was agitated for 1 h. Subsequently, isopropyl alcohol (10 mL) was added and the initially blue solution gradually changed to red purple. The prepared solution was filtered using filter paper. Then, the filtrate evaporated to obtain gold nanoparticles under reduced pressure, which were washed with toluene, acetone, and water to remove the traces of fullerene oxide and K2CO3. After centrifugation of the solution for 30 min, the sediment was dried to obtain the gold nanoparticles.
4. Catalytic activity of the synthesized gold nanoparticles in the reduction of 4-nitroaniline
In order to achieve the reduction of 4-nitroaniline, NaBH4 (10 mg) was added to a 4-nitroaniline aqueous solution (15 mL, 80 mM) in a 30 mL vial. Then, gold nanoparticles powder (3 mg) was added to the 4-nitroaniline solution and mixed using a magnetic bar. The progress of the reduction of 4-nitroaniline to 1,4-phenylenediamine was monitored at various time periods using a UV-vis spectrophotometer.
5. Kinetics study of the reduction of 4-nitroaniline
Langmuir-Hinshelwood model was used to evaluate the catalytic activity of the synthesized gold nanoparticles. The kinetic equation is as follows:
C0 is the initial concentration of 4-nitroaniline and C is the concentration at stirring time t. After addition of the synthesized gold nanoparticles to a stirring solution containing NaBH4 and 4-nitroaniline, UV-vis spectra were recorded every minute.
Results and Discussion
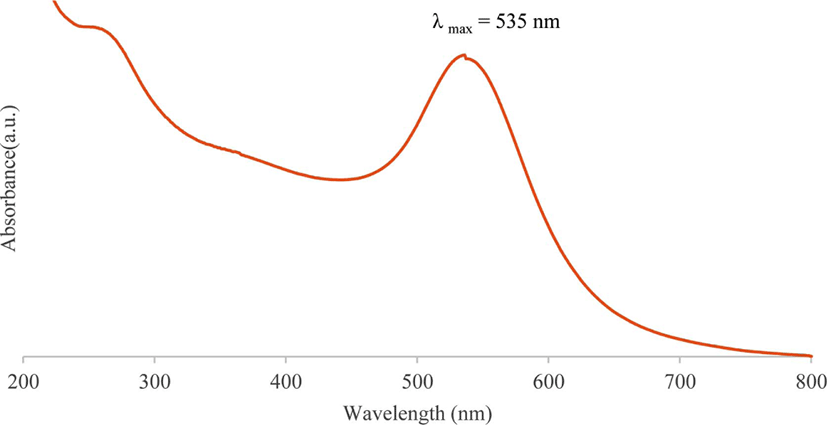
The presence of metallic gold nanoparticles in colloidal state in an aqueous solution can be easily detected by a spectro-photometric analysis. Figure 1 shows the UV-vis spectrum of the gold nanoparticles synthesized with fullerene oxide, which has a surface plasmon band peak in the range between 530 and 540 nm.27,28
Figure 1.
UV-vis spectrum of synthesized gold nanoparticles using fullerene oxide [C60(O)n, n ≥ 1]
Download Original Figure
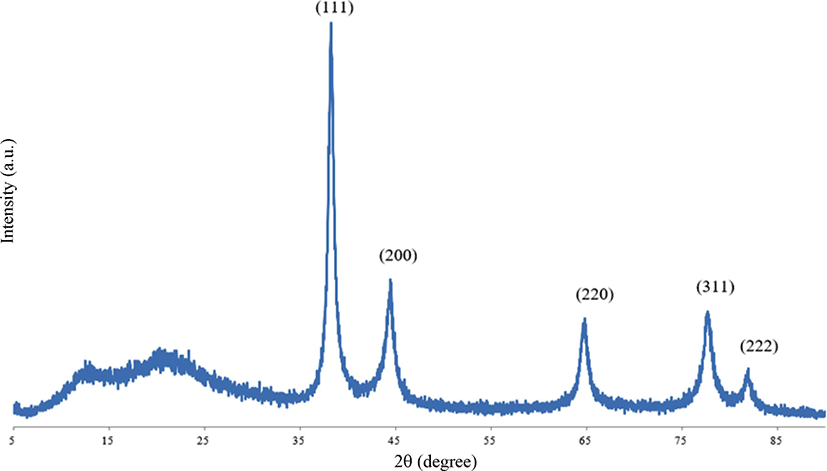
As illustrated in Figure 2, the XRD patterns showed 2θ values of 38.27°, 44.39°, 64.73°, 77.76°, and 81.94° matching to the (111), (200), (220), (311), and (222) as a reflective plane. The XRD pattern indicates that the gold nanoparticles crystallized in a face centered cubic (fcc) lattice structure and were successfully synthesized using fullerene oxide [C60(O)n, n ≥ 1] as the catalyst.29-31
Figure 2.
XRD patterns of synthesized gold nanoparticles using fullerene oxide [C60(O)n, n ≥ 1].
Download Original Figure
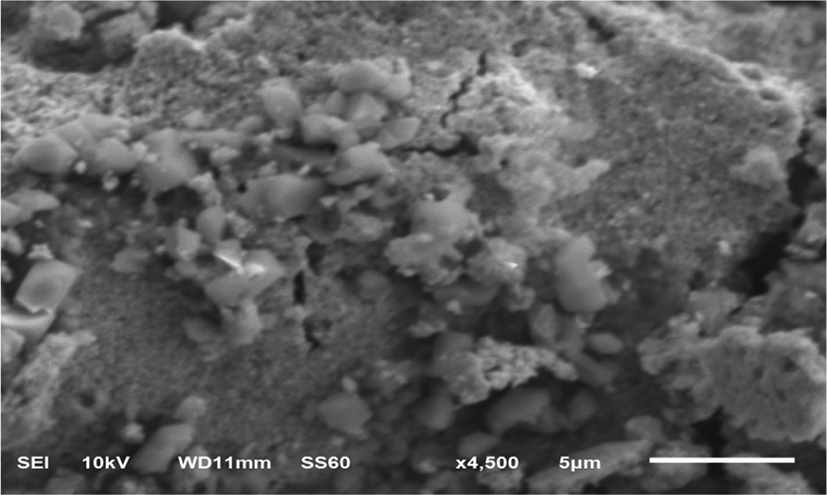
Figure 3 describes the SEM image of the synthesized gold nanoparticles, suggesting that they possess an octahedral-like shape.
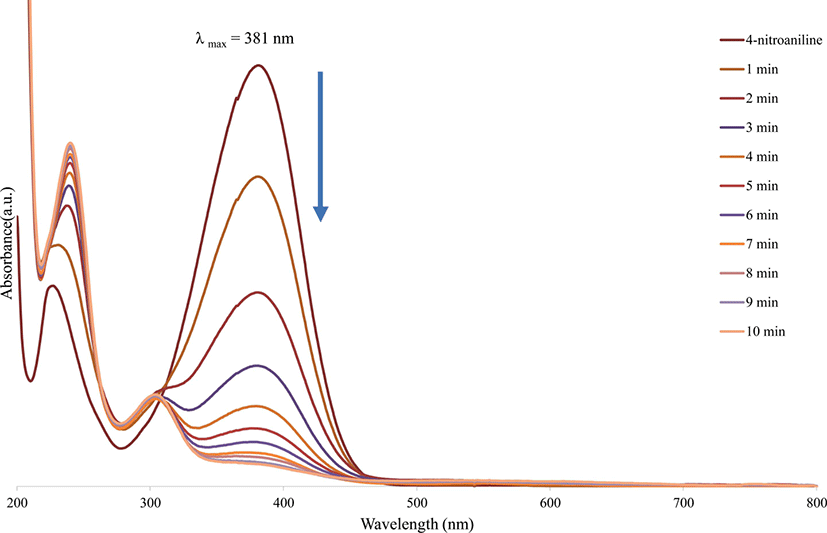
Figure 4 illustrates the UV-vis spectra of 4-nitroaniline, which was measured at one minute time interval during the catalytic reduction by the gold nanoparticles in the presence of sodium borohydride. The addition of gold nanoparticles to an aqueous mixture containing NaBH4 and 4-nitroaniline resulted in a prompt decrease in the absorption band at 381 nm and a concomitant increase in the absorption band at 240 nm, with the advent of a new absorption band at 303 nm. Due to the reduction of 4-nitroaniline, the concentration of 4-nitroaniline gradually decreased. It is apparent that the gold nanoparticles act as catalysts to accelerate the reduction of 4-nitroaniline. Moreover, gold nanoparticles were effective as reduction catalysts for 4-nitroaniline in the presence of NaBH4.32
Figure 4.
UV-vis spectra of 4-nitroaniline reduction with gold nanoparticles as catalyst in the presence of NaBH4.
Download Original Figure
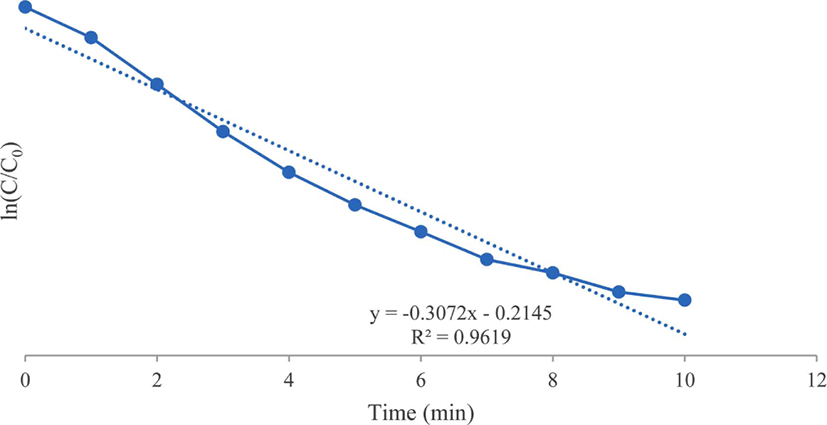
As shown in Figure 5, the reduction of 4-nitroaniline by NaBH4 and the gold nanoparticle catalyst follows pseu-do-first-order kinetics. The reaction rate was established by equation ln(C/C0) = −k·t, where k is the rate constant, C/C0 is the concentration ratio for different measuring states at the time t and initial state.33
Figure 5.
Kinetics of 4-nitroaniline reduction using synthesized gold nanoparticles as catalyst.
Download Original Figure
Conclusions
In summary, gold nanoparticles were successfully synthesized in the presence of fullerene oxide [C60(O)n, n ≥ 1], possesses an octahedral-like shape. The as-prepared gold nanoparticles were tested as catalysts for promoting the reduction of 4-nitroaniline to 1,4-phenylenediamine, and they exhibited excellent catalytic activity in this model reaction. The reductive reaction of 4-nitroaniline was identified to follow the pseudo-first order kinetics at room temperature.
Acknowledgements
This study was supported by Sahmyook University research funding in Korea.
References
X. Zhang, J. Zhang, W., P. H. Xiang, and J. Qiao, “Fabrication of graphene-fullerene hybrid by self-assembly and its application as support material for methanol electrocatalytic oxidation reaction”,
Appl. Surf. Sci.,
440, 477 (2018).


H. Imahori and Y. Sakata, “Fullerenes as novel acceptors in photosynthetic electron transfer”,
Eur. J. Org. Chem.,
1999, 2445 (1999).


H. J. Hwang, K. R. Byun, J. Y. Lee, and J. W. Kang, “A nanoscale field effect data storage of bipolar endo-fullerenes shuttle device”,
Curr. Appl. Phys.,
5, 609 (2005).


F. Cataldo, “Synthesis of silver nanoparticles by the action of heavy ozonized C
60 fullerene on silver nitrate solutions”,
Fuller. Nanotub. Car. N.,
23, 523 (2015).


Y. Zhou, C. Y. Wang, Y. R. Zhu, and Z. Y. Chen, “A novel ultraviolet irradiation technique for shape-controlled synthesis of gold nanoparticles at room temperature”,
Chem. Mater.,
11, 2310 (1999).


T. K. Sau, A. Pal, N. R. Jana, Z. L. Wang, and T. Pal, “Size controlled synthesis of gold nanoparticles using photochemically prepared seed particles”,
J. Nanopart. Res.,
3, 257 (2001).


C. L. Nehl and J. H. Hafner, “Shape-dependent plasmon resonances of gold nanoparticles”,
J. Mater. Chem.,
18, 2415 (2008).


M. A. Wall, S. Harmsen, S. Pal, L. Zhang, G. Arianna, J. R. Lombardi, and M. F. Kircher, “Surfactant-Free Shape Control of Gold Nanoparticles Enabled by Unified Theoretical Framework of Nanocrystal Synthesis”,
Adv. Mater.,
29, 1605622 (2017).




C. J. Orendorff, T. K. Sau, and C. J. Murphy, “Shape-dependent plasmon-resonant gold nanoparticles”,
Small,
2, 636 (2006).



B. Yang, J. Chou, X. Dong, C. Qu, Q. Yu, K. J. Lee, and N. Harvey, “Size-Controlled Green Synthesis of Highly Stable and Uniform Small to Ultrasmall Gold Nanoparticles by Controlling Reaction Steps and pH”,
J. Phys. Chem. C,
121, 8961 (2017).


J. Zhang, H. Liu, Z. Wang, and N. Ming, “Shape-selective synthesis of gold nanoparticles with controlled sizes, shapes, and plasmon resonances”,
Adv. Funct. Mater.,
17, 3295 (2007).


E. Hao, R. C. Bailey, G. C. Schatz, J. T. Hupp, and S. Li, “Synthesis and optical properties of “branched” gold nanocrystals”,
Nano Lett.,
4, 327 (2004).


C. L. Nehl, H. Liao, and J. H. Hafner, “Optical properties of star-shaped gold nanoparticles”,
Nano Lett.,
6, 683 (2006).



M. Shen, W. F. Chen, Y. Sun, and C. G. Yan, “Synthesis and characterization of water-soluble gold colloids stabilized with aminoresorcinarene”,
J. Phys. Chem. Solids,
68, 2252 (2007).


T. A. El-Brolossy, T. Abdallah, M. B. Mohamed, S. Abdallah, K. Easawi, S. Negm, and H. Talaat, “Shape and size de-pendence of the surface plasmon resonance of gold nanoparticles studied by Photoacoustic technique”,
Eur. Phys. J. Spec. Top.,
153, 361 (2008).


M. J. Ashley, M. R. Bourgeois, R. R. Murthy, C. R. Laramy, M. B. Ross, R. R. Naik, and C. A. Mirkin, “Shape and size control of substrate-grown gold nanoparticles for surface-enhanced raman spectroscopy detection of chemical analytes”,
J. Phys. Chem. C,
122, 2307 (2018).


C. Morasso, D. Mehn, R. Vanna, M. Bedoni, E. Forvi, M. Colombo, and F Gramatica, “One-step synthesis of star-like gold nanoparticles for surface enhanced Raman spectroscopy”,
Mater. Chem. Phys.,
143, 1215 (2014).


H. Chen, X. Kou, Z. Yang, W. Ni, and J. Wang, “Shape-and size-dependent refractive index sensitivity of gold nanoparticles”,
Langmuir,
24, 5233 (2008).



C. R. Holkar, A. J. Jadhav, D. V. Pinjari, N. M. Mahamuni, and A. B. Pandit, “A critical review on textile wastewater treatments: possible approaches”,
J. Environ. Econ.
Manag.,
182, 351 (2016).



M. R. Langille, M. L. Personick, J. Zhang, and C. A. Mirkin, “Defining rules for the shape evolution of gold nanoparticles”,
J. Am. Chem. Soc.,
134, 14542 (2012).



M. L. Personick, M. R. Langille, J. Zhang, and C. A. Mirkin, “Shape control of gold nanoparticles by silver underpotential deposition”,
Nano Lett.,
11, 3394 (2011).



L. Minati, F. Benetti, A. Chiappini, and G. Speranza, “One-step synthesis of star-shaped gold nanoparticles”,
Colloids Surf. A Physicochem. Eng. Asp.,
441, 623 (2014).


M. Wang, B. De Vivo, W. Lu, and M. Muniz-Miranda, “Sensitive Surface-Enhanced Raman Scattering (SERS) Detection of Nitroaromatic Pollutants in Water”,
Appl. Spectrosc.,
68, 784 (2014).



A. A. Al-Kahtani, T. Almuqati, N. Alhokbany, T. Ahamad, M. Naushad, and S. M. Alshehri, “A clean approach for the reduction of hazardous 4-nitrophenol using gold nanoparticles decorated multiwalled carbon nanotubes”,
J. Clean. Prod.,
191, 429 (2018).


C. Umamaheswari, A. Lakshmanan, and N. S. Nagarajan, “Green synthesis, characterization and catalytic degradation studies of gold nanoparticles against congo red and methyl orange”,
J. Photochem. Photobiol. B,
178, 33 (2018).



J. W. Lee, Y. M. Lee, B. E. Park, and W. B. Ko, “Efficient Method for the Cleavage of Fullerene Oxides with Several Aromatic Amines under Ultrasonic Irradiation”,
Elast. Compos.,
42, 1 (2007).

W. Haiss, N. T. Thanh, J. Aveyard, and D. G. Fernig, “Determination of size and concentration of gold nanoparticles from UV-Vis spectra”,
Anal. Chem.,
79, 4215 (2007).



N. R. Jana, L. Gearheart, and C. J. Murphy, “Seeding growth for size control of 5-40 nm diameter gold nanoparticles”,
Langmuir,
17, 6782 (2001).


Y. Chen, X. Gu, C. G. Nie, Z. Y. Jiang, Z. X. Xie, and C. J. Lin, “Shape controlled growth of gold nanoparticles by a solution synthesis”,
Chem. Commun.,
33, 4181 (2005).



Y. Sun and Y. Xia, “Shape-controlled synthesis of gold and silver nanoparticles”,
Science,
298, 2176 (2002).



Y. Shao, Y. Jin, and S. Dong, “Synthesis of gold nanoplates by aspartate reduction of gold chloride”,
Chem. Commun.,
9, 1104 (2004).



C. Deraedt, L. Salmon, S. Gatard, R. Ciganda, R. Hernandez, J. Ruiz, and D. Astruc, “Sodium borohydride stabilizes very active gold nanoparticle catalysts”,
Chem. Commun.,
50, 14194 (2014).



S. S. R. Gupta, M. L. Kantam, and B. M. Bhanage, “Shape-selective synthesis of gold nanoparticles and their catalytic activity towards reduction of pnitroaniline”,
Nano-Struct. Nano-Objects,
14, 125 (2018).