Introduction
Plastics are broken down through mechanical fragmentation, high temperatures, ultraviolet (UV) degradation, and biological digestion, and the resulting fragments are classified as microplastics when their size is less than 5 mm.1 Microplastics are classified into primary and secondary microplastics based on their origin. Primary microplastics are manufactured in microscopic sizes from the beginning, while secondary microplastics are generated through fragmentation caused by the weathering of larger plastic products. Weathered microplastics tend to have more diverse shapes and sizes than primary microplastics and can exert environmental2 and biological impacts by adsorbing and transporting toxic substances on their surfaces or exhibiting cytotoxicity.
Each type of plastic has a unique molecular structure and physical properties, which directly influences its degradation behavior and microplastic formation mechanisms during weathering. Overall, UV radiation from sunlight plays a major role by inducing photo-oxidation in polymer chains, which is often followed by mechanical abrasion or thermal stress, ultimately leading to the generation of secondary microplastics. Fourier Transform Infrared Spectroscopy (FT-IR) and Raman spectroscopy are the most commonly used techniques for chemically identifying microplastics. Raman scattering is an inelastic scattering process, and the scattering of photons is caused by changes in the polarizability of molecules. Molecules exhibit characteristic vibrational patterns depending on their chemical bonds and functional groups, and these patterns appear as distinct peaks in the spectrum, allowing the sample to be identified as a fingerprint. Raman spectroscopy is capable of analyzing very small microplastic particles, even those smaller than 1 μm, and is non-destructive, requiring no special sample preparation to obtain a spectrum. Moreover, it is not sensitive to moisture, allowing for unrestricted measurement of various samples.
Weathering of plastics affects both the crystalline and amorphous regions of polymers, and this is observed in Raman spectra as peak shifts, peak broadening, and changes in intensity. Notably, weathering pathways differ depending on exposure to natural or artificial seawater and air, and such environment-dependent changes can be detected using Raman spectroscopy. Additionally, oxygen-containing functional groups are formed during weathering, and their presence can be qualitatively and quantitatively assessed based on peak positions and intensities. Structural and chemical changes on the surface can also be analyzed to evaluate the mobility, adsorption behavior, and biological effects of microplastics in the environment, making Raman spectroscopy one of the most widely used analytical techniques in this field. In the case of IR analysis, quantitative evaluation is possible by monitoring polar functional groups (such as the O–H band) that are introduced during the weathering process. By using Attenuated Total Internal Reflection Fourier Transform Infrared spectroscopy (ATR-FTIR), spectral changes can be tracked over time, and statistical techniques such as Principal Component Analysis (PCA) can be applied to quantify the degree and type of weathering. Furthermore, carbonyl groups are formed due to environmental stress during weathering, leading to an increase in the Carbonyl Index (CI) value. IR-based analysis is advantageous for quantifying and comparing the extent of weathering and it has proven to be effective in identifying the degree of microplastic weathering.3 Therefore, this review examines studies on the weathering of eight different types of plastics, with a focus on the use of Raman and IR spectroscopy
Characteristics of Each Plastic Type and the Effects of Weathering Mechanisms
Polypropylene (PP) is a polyolefin similar to polyethylene (PE), but it differs significantly in that it has methyl side groups attached to its carbon backbone. Unlike PE, in which the backbone consists of secondary carbon atoms, the carbon atoms in the PP backbone are tertiary, making PP more susceptible to photochemical degradation than PE.4 The C–H bonds of these tertiary carbon atoms are more easily converted into radicals under UV exposure, leading to faster oxidation reactions. In fact, studies have shown that although PP is less reactive than polystyrene (PS) with aromatic structures, it is more susceptible to photo-oxidation than PE. Many experiments have reported that PP exhibits stronger signs of aging in a shorter period compared to PE.5 The initial stage of PP weathering is characterized by rapid oxidation induced by UV exposure. Even within a short period of initial UV irradiation, the CI on the PP surface increases sharply. According to the report by Jang et al. (2017), the CI of PP showed a rapid increase within the first two months of exposure, followed by a temporary plateau.6 This suggests that PP is highly sensitive to initial UV exposure, leading to rapid oxidation, and that the oxidized products formed may temporarily inhibit further oxidation, resulting in a short-term plateau. In contrast, the CI of PE increases more gradually and linearly, providing a clear distinction.
When photo-oxidation introduces a large number of oxygen-containing defects into the PP chains, the polymer becomes significantly more brittle. As the molecular weight decreases and the crystalline structure weakens due to aging, the material’s resistance to impact is drastically reduced. Consequently, under appropriate mechanical stress, weathered PP readily fragments into micro-sized particles.6
In a study by Zjacić et al. (2024), PP films were subjected to photo-aging and then ground for toxicity and related evaluations. The results revealed that photo-aging led to chain scission, which in turn induced surface cracking and increased the brittleness of the polymer. Long-term environmental behavior also supports this trend.7 In an 18-month artificial weathering experiment conducted by Reineccius et al. (2023), among five types of microplastics (High-Density Polyethylene (HDPE), PP, PS, Polyethylene Terephthalate (PET), and Polyvinyl chloride (PVC)), PP exhibited the earliest visible cracks and initial fragmentation, observed as early as the 9-month mark. It was reported that PP showed signs of degradation earlier than PE.8 In a study by Wang et al. (2025), PE, PP, and PS samples were buried in a coastal wetland in China for 24 months to observe outdoor weathering. The results showed that PS degraded the fastest, followed by PP, and then PE, indicating a degradation susceptibility order of PS > PP > PE. In this study, the annual increase in the CI of PP was approximately 61.8%, which was higher than that of PE but lower than that of PS, supporting the observed ranking.5 As an additional effect of PP weathering, environmental impacts due to the leaching of additives or oxidative degradation products have also been reported. Reineccius et al. (2023) observed that weathered PP pellets caused a decrease in the pH of surrounding water bodies, which was presumed to result from the leaching of acidic additives or degradation products (such as HCl) contained in the PP.8 In addition, the leachates from photo-aged PP films and pristine PP films were analyzed for organic content and metal concentrations.7
This is presumed to be due to organic additives or metal catalysts used during the synthesis of PP, and it was shown that discarded packaging PP released most metal elements more strongly, even at trace concentrations, compared to its pristine counterpart.
PE is a long-chain hydrocarbon polymer without aromatic rings and lacks functional groups that strongly absorb UV light. This structure makes PE more resistance to photochemical degradation compared to polymers with more reactive bonds. The carbon-carbon (C–C) bonds in PE have a bond dissociation energy of about 96 kcal/mol, higher than that of PP or PS, so more UV energy is needed to break PE chains. As a result, pure PE, without impurities or additives, degrades slowly under sunlight.6
Over time, howver, prolonged UV exposure causes PE to oxidize. Oxygen is incorporated into the polymer, forming carbonyl groups (such as ketones, aldehydes, and carboxylic acids), The CI-the ratio infrared absorbance of the carbonyl band to a reference band is commonly used to track PE weathering. For PE, the CI increases almost linearly with UV exposure, indicating a steady, gradual oxidation.6 This process breaks the polymer chains, making material more brittle. As the amorphous, weaker regions are oxidazed and removed first, the overall crystallinity of the material increases.8
Jang et al. (2017) found that low-density PE pellets abraded with sand for two months without prior UV exposure produced only about 9 microfragments per pellet—a very small amount compared to more degradable plastics. However, when PE was pre-oxidized by UV light and then abraded, about after 20 fragments formed per pellet. Under the same conditions, PP generated over 6,000 fragments.6
Both field and laboratory studies support these results. In an 18-month artificial beach weathering test with UV, water, and sand, HDPE pellets showed no measurable mass loss or microfragments formation.8 Similarly, in a two-year coastal wetland exposure, PE microplastics showed the lowest surface oxidation and physical degradation compared to PP and PS.5 These finding suggest that PE is relatively stable.
Research also showed that the IR spectra differed between artificial seawater and real marine environments, indicating that lab conditions do not fully mimic natural processes (Figure 1).
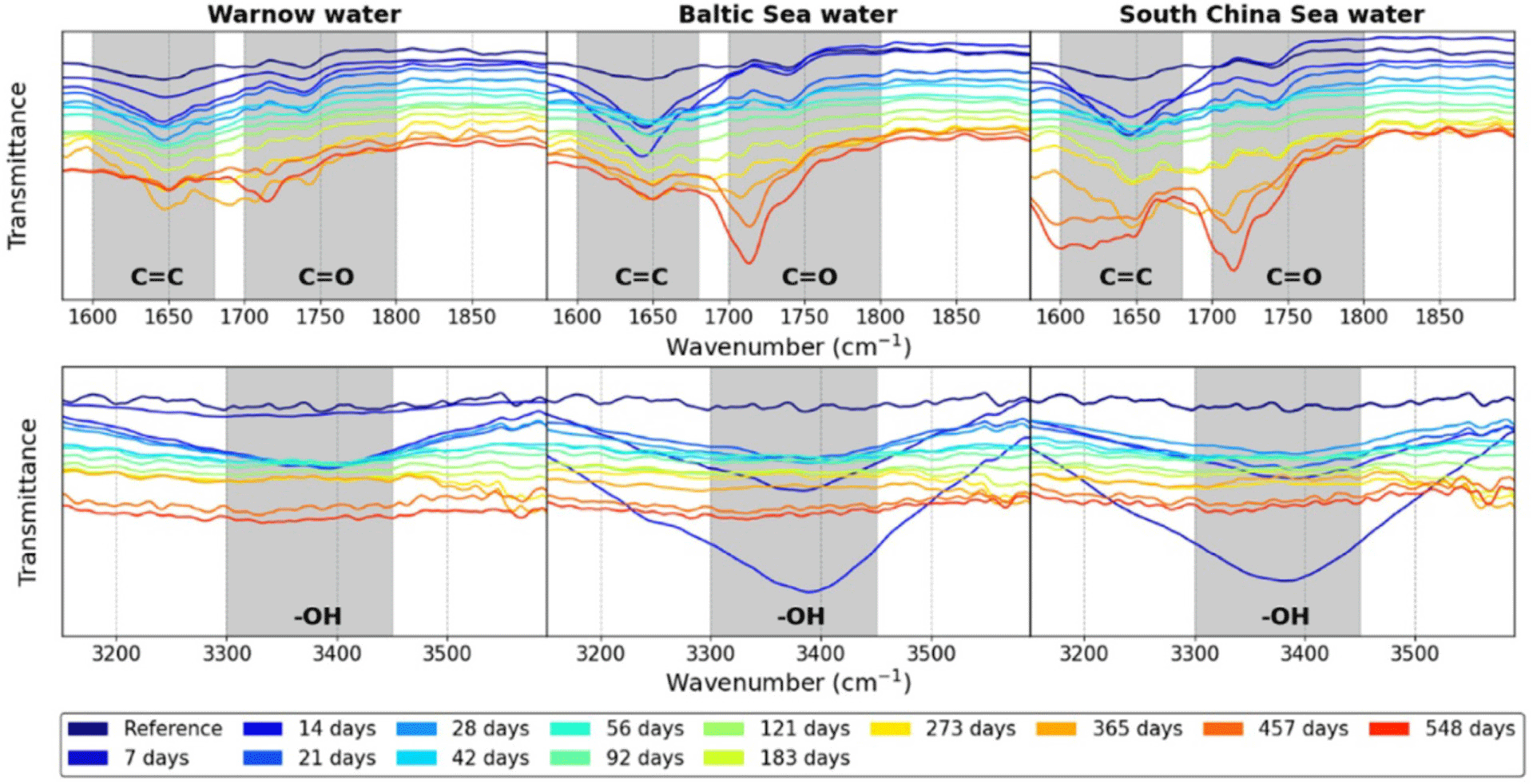
As PE degrades, it can form microplastics and nanoplastics through oxidation, but many of the smallest oxidation products may dissolved as organic molecules. A study cited by Sunil et al. (2024), Royer et al. (2018) reported that common plastics emit greenhouse gases when exposed to sunlight. PE was identified as a source of methane and ethylene. Gas release increases when cracks form and new surfaces are exposed. Although the total greenhouse gas emitions from PE are negligible compared to fossil fuels, this suggests that aging plastic waste could gradually release climate-relevant gases into the atmosphere, potentially contributing to climate change.9
PS shows different photochemical behavior than polyolefins due to the aromatic rings in every repeating unit. PS absorbs more UV radiation than PE or PP because of its benzene rings. Short-wavelength UV can be absorbed and trigger excited-state reactions in the aromatic rings or nearby backbone. The bond dissociation energy of PS (about 90 kcal/mol at 318 nm) is between that of PE and PP, meaning PS bonds break under weaker UV than PE, but stronger than PP.
Under UV, PS undergoes both chain scission and cross-links. Benzene rings can form new bonds between polymer chains when exposed to radiation. Initial photo-oxidation introduces hydroxyl and carbonyl groups to the aromatic rings or the aliphatic backbone. Wang et al. (2025) found that PS microplastics in coastal wetlands had the highest annual increase in CI compared to PE and PP. The CI of PS increased by about 61–76% per year, much highere than PE or PP, indicating greater oxidation. This is due to the PS’s aromatic structure, which absorb more UV and generates reactive oxygen species.5 Reineccius et al. (2023) also noted that PS pellets showed distinct yellowing after several months of UV exposure.8
The yellowing became more pronounced over time and was linked to the formation conjugated chromophores. This was a unique to PS, as PE and PP rarely show significant color change during weathering. Thus, yellowing is a characteristic sign of PS weathering and reflects underlying chemical changes.
In the 18-month experiment, PS did not produce visible fragments like PP. Its surface became rough and oxidized, but no significant cracking or breakage was seen in solid PS pellets. This may be due to the crosslinkingt, which creates a stabilized surface layer that helps the material stay intact even during oxidation.
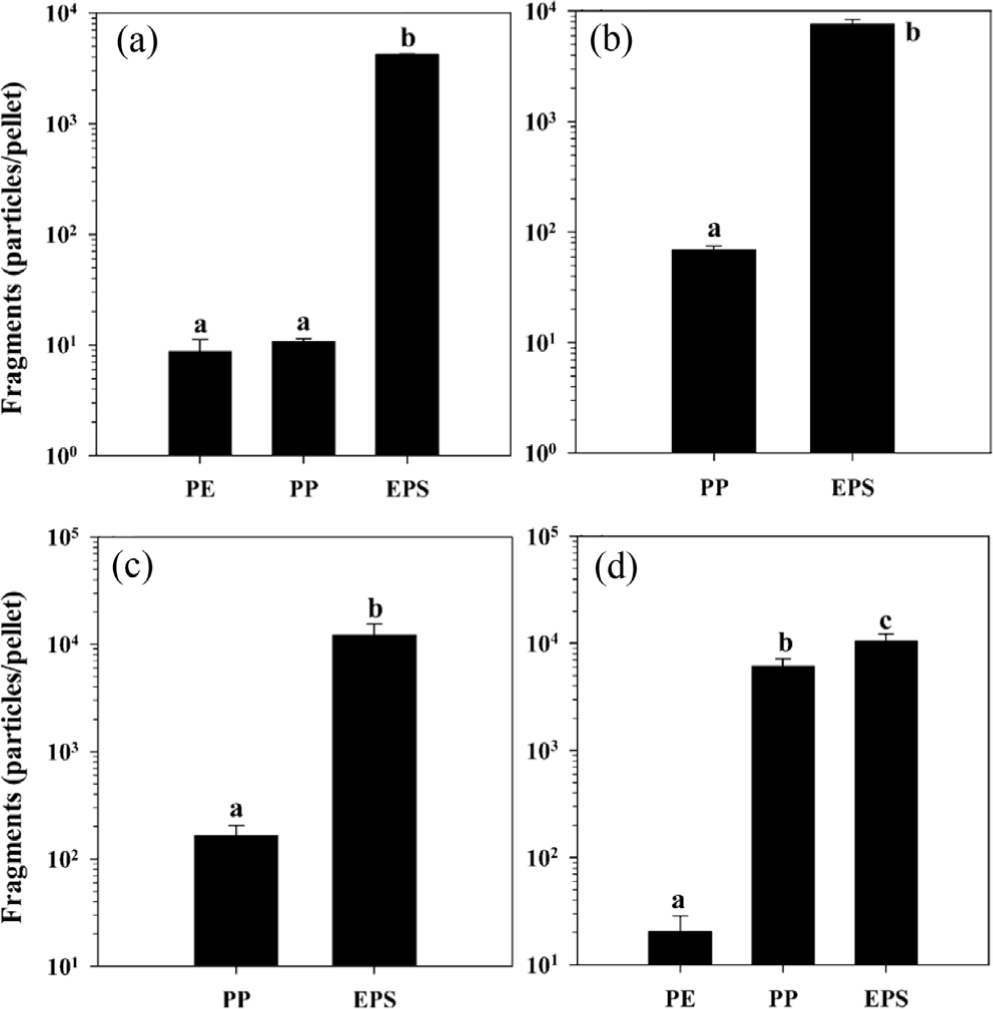
However, with mechanical stress or in Extended Polystyrene (EPS), PS fragments easily. EPS is made by foaming PS with gas, crating a matrix of thin-walled spheres, making it brittle and easily to break. Its high surface area makes it especially prone to fragmentation. Jang et al. (2017) showed that simply agitating EPS with sand for two months generated aboud 4,220 fragments per pellet. With added UV exposure, EPS fragmentation became even more the same conditions. with added UV exposure, EPS fragmentation became even more server-about 12,152 fragments per pallet. Much of the pellet mass disappeared, indicating breakdown into unrecoverable micro-sized particles. Even UV alone caused visible micro-fragmentation at the EPS surface. The porous structure of EPS allows deeper UV penetration and oxygen flow, enabling oxidation throughout the foam. Cracks spread easily trough the thin cell walls, which then break into small fragments.6 Solid PS takes much longer to oxidize and fragment, but EPS fragments rapidly regardless of UV, making it a major source of microplastics. Rigid PS also eventually breaks under mechanical stress.
From an analytical perspective, the CI can be used for PS, but with care. PS initially lacks carbonyl peaks but has characteristic aromatic C=C stretching near 1600 cm−1. As oxidation progresses, new peaks appear around 1710–1720 cm−1. Researchers use the ratio of absorbance at 1715 cm−1 to 1450 cm−1 (ring vibrations) to calculate CI. In Jang’s study, the ratio at 1720/1450 cm−1 was used for EPS.6 Interestingly, after prolonged UV exposure, the CI of EPS decreased, attributed to fragmentation and loss of the most oxidized layers. This shows a challenge in tracking PS weathering: once fragmentation starts, oxidized material is lost as free particles. However, these small particles still contain carbonyl and hydroxyl groups from prior oxidation.
The environmental impact is significant. EPS can rapidly vast amount of micro- and nanoparticles, polluting soil and water. Lozano et al. (2024) found that photo-degraded PS foam reduced soil aggregation and altered microbial activity.10
EPS was found to be markedly more susceptible to fragmentation compared to PE and PP under identical abrasion conditions. According to the results summarized in Figure 2, after two months of mechanical abrasion without prior UV exposure, the number of microplastic fragments generated per pellet was approximately 9 for PE, 11 for PP, but exceeded 4,000 for EPS. Furthermore, after 6 months of UV pre-exposure, fragmentation of EPS increased drastically, yielding over 12,000 fragments per pellet—far greater than that observed for both PE and PP under the same conditions. These data clearly demonstrate that EPS has a much higher propensity to generate microplastics even in the absence of photo-oxidative preconditioning, and that environmental weathering further amplifies this fragmentation process. The degraded foam become more hydrophilic due to oxygen-containing groups, reducing its ability to bind soil particles and thus lowering soil aggregation. The researchers suggested that the presence of degraded foam may stimulate microbial activity, as seen by increased soil respiration, possibly due to the leaching of degradation byproducts. Another study (Rassaei, 2023, cited in Sunil, 2024) reported that degraded PS in soil increased methane emissions,9 possibly by affecting microbial communities or releasing bioavailable organic carbon. These effects are still being studied, but it is clear that weathered PS behaves differently from pristine PS due to its new reactice functional groups.
In water, solid PS has a slightly higher density than water (about 1.05 g/cc), so fragments tend to sink unless buoyed is biofilms or other factors. EPS always floats at first. Solid PS fragments are more likely to sink once detached. However, PS debris is found throughout the water column, from surface to sediment. Because it can break down into very small particles, PS has high potential for biological uptake and transfer through the food web. In summary, PS weathers via relatively rapid chemical oxidation, but the physical fragmentation longer due to depends on its form. EPS is one of the fastest materials to generate secondary microplastics, breaking down with minimal energy. Solid PS resists fragmentation longer due to cross-linking but eventually produced microplastics under abrasion. The aromatic nature of PS means it will not remain intact in the environment indefinitely, ultimately degrading and contributing to microplastic pollution.
Polyesters refer to a class of polymers with ester bonds in their main chains, but in general, the most commonly observed polyester in the environment is PET.11 PET has a structure composed of benzene rings and ester linkages and is widely used in products such as plastic bottles and textiles. Due to the presence of aromatic rings, it exhibits greater UV stability than polyolefins. Additionally, because carbonyl bonds are inherently present in its molecular structure, PET shows a relatively high initial CI in FT-IR analysis. Therefore, while there may be limitations in quantifying additional carbonyl formation during weathering, relative changes can still be observed.12
PET is known to be a polymer with relatively good resistance to UV light. In fact, PET fibers are often used as outer layers in marine gear to compensate for the UV vulnerability of aromatic polyamides(PA), demonstrating that PET exhibits only a gradual decline in mechanical strength under UV exposure.13 The photodegradation of PET primarily affects the ester bonds, leading to chain scission. When PET is exposed to ultraviolet (UV) light in the presence of oxygen, photo-oxidative hydrolysis can occur. This process results in the cleavage of ester linkages, producing carboxyl and hydroxyl end groups.14 Additionally, further oxidation can lead to the formation of aldehyde or vinyl end groups. However, such processes require relatively harsh conditions. In a controlled experiment, Lozano and colleagues (2024) found that PET films exhibited very slow signs of degradation under UV exposure, requiring a doubling of exposure time to obtain measurable FT-IR changes. Even then, the changes were minimal—the strong carbonyl peak of PET did not significantly decrease, and new peaks did not emerge rapidly—confirming PET’s high photostability compared to many other plastics.10
In artificial weathering experiments, PET often exhibits surface changes such as increased surface oxidation and a reduction in molecular weight at the surface. However, actual fragmentation of PET is rarely observed in short- and medium-term studies. In the 18-month beach simulation study by Reineccius et al. (2023), PET pellets did not produce detectable surface fragments, and only a few particles were found in the filtrate. This may be due to mild hydrolysis causing relaxation and micro-scale peeling of the surface layer, but no significant mass loss or cracking was observed. The researchers noted that, unlike PP, both PVC and PET tended to become worn down or smoothed rather than forming pits or indentations.8 Similarly, Wang et al. (2025) reported that while PET in the wetland environment underwent degradation, it did so more slowly than polyolefins and showed minimal fragmentation over the course of two years.5
Although PET does not fragment rapidly, it can undergo slow hydrolysis under specific environmental conditions. In a study on PET hydrolysis using seawater, it was estimated that complete depolymerization of PET at 30°C would take approximately 162 years in the world’s oceans.15 In addition, PET can undergo some degree of degradation in compost or soil environments with high humidity and the presence of microorganisms.16 However, the greatest concern with PET is that it remains as large plastic debris for extended periods, leading to long-term persistence in the environment. In this research context, PET is relatively resistant to weathering but undergoes slow oxidation and shows minimal degradation over several months to years. Changes in the CI are minor, and the formation of measurable microplastics is not typically observed in short-term tests. Therefore, it is important to note that longer-term studies are necessary to fully understand its degradation behavior.
Advanced vibrational spectroscopic techniques—such as ATR-FTIR, synchrotron FT-IR mapping, and high-resolution Raman imaging—have enabled the detection of even subtle and spatially heterogeneous chemical changes occurring in weathered PET [2]. For example, surface analyses using these methods have shown the formation of chemically modified layers characterized by increased absorbance in the ester C=O region (~1715–1730 cm−1) and O–H stretching region (3300–3500 cm−1) [2]. Spatially resolved Raman microscopy provides direct visualization of oxidation hotspots as well as gradual changes in aromatic ring structures at the microplastic surface [2]. Under environmental conditions such as elevated temperature or high pH, PET can undergo hydrolytic cleavage into terephthalic acid and ethylene glycol, as confirmed by chromatographic and NMR analyses [1]. Long-term simulations of PET weathering and controlled field studies further confirm that, although PET is highly resistant to bulk fragmentation and mass loss, localized chemical and microstructural transformations do occur at the surface and can be tracked with high sensitivity using these analytical techniques [1-3]. Overall, the environmental degradation and transformation of PET remain gradual processes.
PVC, commonly used in pipes, vinyl siding, and synthetic leather, is characterized by its unique structure as a chlorinated polymer.17 PVC ranks third in global production volume after PE and PP and has a wide range of applications, but it is known to have a relatively low detection frequency in environmental samples.18 Fernandez-Gonzalez et al. (2022) identified the main reasons for this as PVC’s low weather resistance and the analytical challenges of identifying it after weathering. They noted that among major polymers, PVC is the most sensitive to UV radiation, and photodegradation plays a critical role in its breakdown. In the early stages of photodegradation, the polymer chain becomes highly susceptible to thermal and photochemical attack due to the formation of unsaturated structures resulting from dehydro-chlorination, leading to a decrease in molecular weight and a brittle state.
The degradation chemistry of this material is governed by the behavior of the C–Cl bond. The primary weakness of PVC lies in the C–Cl bond, which can undergo uniform scission or, more commonly, dehydrochlorination. When PVC is heated or exposed to radiation, chlorine atoms are lost as HCl, leaving carbon–carbon double bonds in the polymer chain. The loss of one HCl molecule makes adjacent monomer units more prone to HCl elimination, resulting in the formation of polyenes.19 These polyenes are responsible for the discoloration observed in aged PVC. The formation of microplastics during PVC weathering can be understood as a process involving photo-oxidation, mechanical weakening, and fragmentation. Initially formed polyene structures through dehydrochlorination undergo further oxidation over time, leading to the introduction of polar functional groups such as carbonyls, along with complex chemical changes such as chain scission and cross-linking.8 If UV exposure and degradation progress sufficiently, these changes can eventually lead to fragmentation into micro-sized particles.
Although various stabilizers and plasticizers are added in large amounts to suppress such photo- and thermal degradation,13,18 severe degradation still occurs when exposure exceeds a certain threshold. Fernandez-Gonzalez et al. (2022) described that PVC microplastics left outdoors for extended periods eventually undergo such severe degradation that their original spectral patterns become barely recognizable.18 When exposed outdoors, PVC is typically stabilized with metal salts or organotin compounds, which bind or neutralize HCl to prevent dehydrochlorination.20 According to Reineccius (2023), PVC pellets exhibited a high calcium carbonate content.8 This serves to neutralize the generated HCl, which may explain why PVC did not exhibit measurable fragmentation or dramatic chemical changes during the 18-month experiment. When examining the weathering products of PVC, plasticizers and heavy metal stabilizers can leach out and disperse into the environment.13 Fawzi H. Jabrail and colleagues (2021) noted that the HCl released during the dehydrochlorination process can cause corrosion of nearby metals and changes in the pH of surrounding soil or water bodies.21
In the study by Reineccius et al. (2023), a rapid decrease in surface contact angle (i.e., increased hydrophilicity) was observed in PVC within the first week, due to the introduction of polar functional groups such as –OH8 This indicates that the PVC surface becomes highly polarized in a very short time. The increase in hydrophilicity is likely due to the release of Cl− ions and hydrophilic oligomers resulting from PVC degradation.
FT-IR, Raman spectroscopy, and X-ray Photoelectron Spectroscopy (XPS) are commonly used to track the weathering of PVC, with Raman spectroscopy being particularly useful for monitoring the formation of polyenes in PVC.8 FT-IR can be used to monitor the increase in C=O and C=C functional groups and to calculate the CI. However, since PVC is originally a polymer that does not contain C=O groups, the interpretation of CI may differ from that of PE and PP. Therefore, Fernandez-Gonzalez et al. (2022) emphasized the need for caution when interpreting the spectra of weathered PVC.18
PA, commonly known as nylons, are polymers with repeating amide bonds (-CONH-) in their backbone. Representative types include nylon-6 and nylon-6,6, which are widely used in fishing lines, nets, and related gear. Due to their ability to form hydrogen bonds, polyamides exhibit excellent mechanical strength and abrasion resistance. However, they are also hygroscopic, meaning they can undergo hydrolysis over time in humid environments.
In the environment, the weathering of PA involves both physical abrasion and chemical degradation. PA fibers in the form of fishing nets or ropes are continuously worn down by physical forces such as waves, sand, and marine organisms, releasing microfibers into the environment, which contributes to the issue of discarded fishing gear, as abandoned nylon nets in the ocean gradually abrade into fine fibers, which can be ingested by aquatic organisms and accumulate on the seafloor.13 In their study on the chemical and physical weathering of fishing nets, Dabrowska et al. (2021) noted that nets made from all types of synthetic fibers eventually release microplastic fibers and additives into the environment.13 From a chemical perspective, PA are relatively stable against UV radiation but can still undergo gradual photo-oxidation and thermo-oxidation. In particular, aromatic PA, while exhibiting excellent strength, are highly susceptible to UV degradation and experience rapid deterioration in physical properties when exposed to sunlight.13 In contrast, aliphatic nylons do not contain aromatic rings, but under UV exposure, oxidation can occur around the amide bonds within the polymer chains, leading to yellowing and reduced strength. However, the primary natural degradation mechanism of PA is hydrolysis.22 The amide bonds in PA can be gradually broken down through nucleophilic attack by water molecules, and this process is particularly sensitive to temperature and pH conditions. In an experiment conducted by Pfohl et al. (2022), where PA were exposed to UV radiation, it was observed that PA not only released micro-sized fragments through photodegradation but also exhibited a tendency for their polymer chains to break down into smaller units, leaching out as water-soluble low-molecular-weight organic compounds.22 This indicates that the weathering of PA can produce not only secondary microplastics and nanoplastics but also soluble residues. In fact, the study revealed that a significant portion of the mass loss in polyamides was detected as soluble fragments smaller than 10 nm, demonstrating that hydrolytic degradation can occur in PA, not just in polymers with ester linkages.
As 3D printing materials are increasingly used in functional textiles and other applications, certain polymers are becoming more likely to enter the environment. TPU is one such polymer, and its durability can vary greatly depending on its composition. Pfohl et al. (2022) systematically evaluated how the chemical structure of TPU affects microplastic generation. They compared TPUs made with aromatic diisocyanates, which form rigid segments, to those based on aliphatic systems. Aromatic TPUs showed significant yellowing and embrittlement under UV exposure, with fractured injection-molded structures leading to rough surfaces and microfragment formation. In contrast, aliphatic TPUs were more stable, exhibiting minimal physical property changes and negligible particle release. Notably, the polyether-aromatic TPU composition generated up to 0.5 mg of nanoplastics per gram of sample after 2,000 hours of weathering, whereas the aliphatic TPU composition did not release any detectable nanoparticles.22 These results suggest that the risk of microplastic pollution in the environment can be mitigated through careful design of polymer structures.
Since TPU products often contain various additives depending on their intended use, the release of hazardous chemicals during weathering must also be considered. In some aromatic TPU products, degradation may lead to the detection of Diphenyl Methane Diisocyanate (MDI)-like structures or amine-based compounds.23 These substances can exhibit toxicity in aquatic environments. In contrast, degradation products of aliphatic TPU are generally considered to be relatively non-toxic.
Bioplastics were developed to address the environmental issues associated with conventional plastics, with their key feature being their ability to biodegrade through microbial activity. However, it has been revealed that bioplastics do not always fully degrade in the environment and can instead break down into microplastics and nanoplastics. This has raised concerns that bioplastics may pose similar environmental risks to those of traditional plastics.24–26 Microplastics derived from bioplastics have been shown to exhibit ecological toxicity similar to that of conventional plastics. For example, when Polylactic Acid (PLA) microplastics were introduced to the marine benthic organism Arenicola marina (lugworm), observed effects included a 1.9-fold increase in metabolic rate (as measured by oxygen consumption), a 30% reduction in feeding activity (indicated by decreased fecal pellet production), and a 1.6-fold decrease in the biomass of microalgae (diatoms).24 Polyhydroxybutyrate (PHB) Plastics have been shown to induce toxicity in freshwater organisms such as Daphnia magna and algae, leading to oxidative stress within cells, damage to cell membranes, and inhibited growth.25
Microplastics derived from bioplastics have in some cases demonstrated a higher adsorption capacity for organic pollutants (e.g., phenanthrene) compared to conventional plastics such as PP, PE, and PS. For instance, Polybutylene Adipate Terephthalate (PBAT) microplastics have been reported to adsorb 50% more phenanthrene, highlighting their potential role as vectors for transporting environmental contaminants.26 These cases suggest that microplastics generated from bioplastics can facilitate the spread of pollutants in the environment. They also indicate that bioplastic-derived microplastics may cause ecological disruption and harmful effects at levels comparable to those of conventional plastics.
Conclusions
This review analyzed recent research trends on plastic weathering with a focus on Raman and IR spectroscopy. Although research on microplastics has surged in response to increased plastic consumption, studies specifically addressing the weathering behavior and environmental transformation of different plastic types remain relatively limited. Plastics differ significantly in their chemical structures, additives, and surface properties depending on the type, which leads to varied chemical and physical changes during weathering, as well as differences in pollutant adsorption behavior in the environment. Therefore, it is increasingly important to conduct detailed analyses of plastic-specific characteristics and to investigate how these properties evolve through weathering.
Weathering, driven by environmental factors such as UV radiation, temperature, and abrasion, causes oxidation, microcracking, and the introduction of new functional groups on plastic surfaces. These changes lead to increased surface area, alterations in hydrophilicity and surface charge, and enhanced pollutant adsorption capacity. Weathered microplastics tend to engage in more complex interactions within the environment, and their ecological impact can vary depending on the plastic type and degree of weathering.
It is also important to recognize the significant differences between artificially weathered plastic samples and real-world plastic waste collected from the environment. Artificially weathered plastics typically undergo controlled and relatively simple weathering processes, whereas environmental plastic waste is exposed over long periods to a wide range of unpredictable factors, including UV radiation, temperature fluctuations, microorganisms, and various pollutants. As a result, environmental samples often show different properties, such as altered chemical structures, surface contamination, and microbial biofilm formation, which pose challenges in directly applying laboratory findings to real-world scenarios. Thus, research using environmental samples must be conducted alongside lab-based studies.
Raman and IR spectroscopy are powerful, non-destructive tools for analyzing the chemical structure and types of microplastics. Recent technological advances have enabled the detection and characterization of ultrafine particles. However, to improve analytical accuracy, and to quantitatively assess and compare the weathering characteristics of various plastic types under real environmental conditions, there is a need for standardized analytical protocols and a broader accumulation of case studies.